Abstract
Developments of technologies for delivery of foreign genes to the central nervous system are opening the field to promising treatments for human neurodegenerative diseases. Gene delivery vectors need to fulfill several criteria of efficacy and safety before being applied to humans. The ability to drive expression of a therapeutic gene in an adequate number of cells, to maintain long-term expression, and to allow exogenous control over the transgene product are essential requirements for clinical application. We describe the use of an adenovirus vector encoding human tyrosine hydroxylase (TH) 1 under the negative control of the tetracycline-sensitive gene regulatory system for direct injection into the dopamine-depleted striatum of a rat model of Parkinson’s disease. This vector mediated synthesis of TH in numerous striatal cells and transgene expression was observed in a large proportion of them for at least 17 weeks. Furthermore, doxycyline, a tetracycline analog, allowed efficient and reversible control of transgene expression. Thus, the insertion of a tetracycline-sensitive regulatory cassette into a single adenovirus vector provides a promising system for the development of successful and safe therapies for human neurological diseases. Our results also confirm that future effective gene replacement approaches to Parkinson’s disease will have to consider the concomitant transfer of TH and GTP-cyclohydrolase transgenes because the synthesis of the TH cofactor tetrahydrobiopterin may be crucial for restoration of the dopaminergic deficit.
Parkinson’s disease (PD) is a severe neurological disease characterized by dysfunction of the dopaminergic nigrostriatal system (1). The major neurochemical manifestation of this disorder is the loss of the neurotransmitter dopamine (DA) in the striatum as a result of the progressive degeneration of the dopaminergic neurons in the substantia nigra. The most common therapy is a pharmacological treatment: dopa is administered to restore striatal DA levels. Although chronic peripheral administration of dopa consistently alleviates the symptoms of the disease in its early stages, its efficacy decreases with clinical progression, and adverse motor complications develop in the long-term (2).
Gene therapy holds much promise for the development of treatment for human neurodegenerative diseases for which, as is the case of PD, current clinical approaches are not satisfactory. Striatal delivery of a transgene encoding tyrosine hydroxylase (TH), the rate-limiting enzyme in the biosynthesis of DA, to supply dopa locally is an alternative to the pharmacological treatment of PD. The first series of investigations in animal models of the disease demonstrated that cells genetically engineered to produce TH implanted into the DA-depleted striatum can partially reverse the animals’ behavioral deficits (3, 4). There is an alternative to this type of ex vivo gene transfer: The conversion of part of the striatal cell population to dopa producing cells may be more efficient and simple. Several exploratory studies suggest that the direct virus vector-mediated delivery of a TH transgene in vivo may be useful for the development of a gene therapy for PD (5–7).
Before such therapy can be applied to humans, adequate gene delivery systems need to be developed. In the past few years, recombinant adenovirus has emerged as a promising tool for direct gene transfer to the brain, where it infects quiescent and dividing cells with high efficiency (8). Currently, much effort is being focused on the amelioration of first-generation adenovirus vectors: The deletion of additional and ultimately all viral genes from the virus backbone should help overcome its immunogenicity, allowing the creation of more efficient and safer gene transfer vehicles (9–12). Another major issue in human gene therapy is the exogenous control over the therapeutic gene product. A vector for clinical application should allow adaptation of the treatment to the needs of the patient and termination of the therapy as necessary. In the case of PD, the local production of adjustable levels of dopa may be essential to attenuate the severe motor fluctuations and dyskinesias associated with chronic dopa treatment (2) and thus improve the clinical response. The development of efficient gene regulatory systems based on specific transcription factors that respond to exogenous drugs has allowed the integration of inducible expression cassettes into gene delivery vectors (13, 14). One particularly thoroughly studied circuit of transcriptional activation uses elements of the tetracycline-resistance operon of Escherichia coli transposon 10 (15, 16) to create two novel eukaryotic transactivators that are controlled either negatively (tet-off system) or positively (tet-on system) by tetracyclines. The efficacy of these transactivators for the regulation of foreign gene expression in a variety of mammalian organs, including the brain, has been demonstrated (17–21).
We recently reported the construction of an adenovirus vector encoding human TH under the negative control of the tet-off regulatory system [AdPGK⋅tet⋅hTH1 (where hTH1 stands for human tyrosine hydroxylase 1)]. AdPGK⋅tet⋅hTH1 was functional in infected human neural progenitor cells both in culture and after transplantation to the rat brain (22). Here we describe the effect of the direct injection of AdPGK⋅tet⋅hTH1 into the DA-depleted striatum of a rat model of PD. The long-term ability of this vector to drive production of TH in striatal cells and its responsiveness to doxycycline (dox) were analyzed. The biochemical and behavioral effects of TH synthesis in the denervated striatum were investigated.
Materials and Methods
6-OHDA Lesion, Intracerebral Injection of AdPGK⋅tet⋅hTH1, and Immunosuppression.
Young adult female Sprague–Dawley rats were used (Charles River Breeding Laboratories, Saint Aubin les Elbeuf, France). Surgery was performed under anesthesia with 800 μl/kg of a 1:1 mixture of ketamine (Virbac animaux de Compagnie, Vissom, France) and Rompun (Bayer, Puteaux Cedex, France). Eight micrograms of 6-hydroxydopamine (6-OHDA) was injected stereotactically as described (23) into the left ascending mesostriatal dopaminergic pathway at a rate of 1 μl/min by using a microinjection pump (CMA/100; Carnegie Medicin, Stockholm). Five to six weeks later, the apomorphine-induced rotational behavior of animals was monitored as described (23) two or three times at 1-week intervals. Animals displaying mean scores of at least 250 rotations in 40 min, corresponding to a >90% depletion of striatal dopamine (24), were selected. The AdPGK⋅tet⋅TH1 stock (3.5 × 1010 plaque-forming units/ml) was diluted in PBS and was injected by using a microinjection pump and a 31-gauge cannula at a rate of 2.5 μl/min into nine intrastriatal sites as described (5). Animals were immunosuppressed with a daily i.p. injection of 20 mg/kg cyclosporin (Novartis, Rueil-Malmaison, France) from 2 days before and until 2 days after virus inoculation. The dose of cyclosporin then was decreased to 10 mg/kg until day 7, after which immunosuppression was stopped. Dox was administered in the drinking water at a concentration of 1 mg/ml.
Qualitative and Quantitative Histological Analyses.
At various times after injection of the virus, animals were perfused and brains processed as described (5). Striatal sections (20 μm) were analyzed by standard immunohistochemical procedures. Primary antibodies were polyclonal anti-TH (Institut J. Boy, Strasbourg, France; 1:3,000 or 1:600 for immunofluorescence), monoclonal anti-nuronal nuclear antigen (NeuN, Chemicon; 1:100), and anti- glial fibrillary acidic protein (GFAP, Dako; 1:100). Secondary antibodies and labeling systems were the Vectastain kit (Vector Laboratories), FITC-conjugated anti-mouse or anti-rabbit IgG (1:100), and cyanin 3-conjugated anti-mouse or anti-rabbit IgG (Caltag, Burlingame, CA, 1:400) for immunofluorescence.
TH+ cells in each animal were counted on blind-coded sections. The total number of TH+ cells was assessed on every 9th coronal section of 112 sections covering the entire anterior-posterior axis of the infected striatal area. Cells were considered as TH+ only if they were clearly identified as immunolabeled cell bodies (objective ×20), with a cell diameter ranging between 8 and 17 μm. To obtain the total number of TH+ cells per infected striatum, raw values were corrected by using Abercrombie’s formula (25), with a mean cell diameter of 12.5 μm.
The percent of TH+ cells expressing the astrocytic marker GFAP or the neuronal marker NeuN was determined by using double fluorescence-labeled striatal sections from three animals and a fluorescence microscope. Four sections 80 μm apart from the striatal region in which TH+ cells were particularly abundant (at least 100 TH+ cells/section) were scored for each animal. On each section, the entire infected area was analyzed at high magnification (objective ×40); each field was examined by using a filter set for FITC to detect TH+ cells and a filter set for cyanin 3 for identification of double-labeled cells. Images were taken on a laser-scanning confocal microscope.
Analysis of Striatal Contents of DA and Metabolites.
Five hours before decapitation, 100 mg/kg of (6R)-5,6,7,8-tetrahydro-l-biopterin (6R-BH4; Schirks Laboratories, Jona, Switzerland) was administered subcutaneously in 1% ascorbic acid. Each striatum was dissected, weighed, and homogenized in 125 μl (6-OHDA-lesioned striatum) or 250 μl (contralateral striata) of 0.1 M HClO4 supplemented with 0.05% Na2S2O5 and 0.05% disodium EDTA. Homogenates were centrifuged (30,000 × g, 20 min, 4°C), and the supernatants were neutralized with 2M K2HPO4/KH2PO4 (pH 7.4) and were supplemented with ascorbate oxydase (10 μg/ml; Boehringer Mannheim). After a second centrifugation as above, 10-μl aliquots of the cleared supernatants were injected into a high-performance liquid chromatography column (Ultrasphere IP, 25 cm, 0.46 cm OD, 5 μm; Beckman Coulter) protected with a Brownee precolumn (3 cm, 5 μm). The mobile phase consisted of 70 mM KH2PO4, 2 mM triethylamine, 0.1 mM disodium EDTA, 1.25 mM octanesulfonic acid, and 21% methanol, adjusted to pH 3.0 with solid citric acid. The elution rate was set at 1 ml/min, and electrochemical quantification of dopa, DA, diydroxyphenylacetic acid, and homovanillic acid (HVA) contents was made as described (26).
Statistical Analyses.
One-factor analysis of variance (ANOVA) was used to distribute animals into statistically balanced groups for equality of rotational scores before adenovirus injection; two-factor repeated measures ANOVA was used to determine differences in rotational asymmetry between groups over time; two-tailed paired t tests were used to compare pre- and postinjection rotational scores within groups over time. Two-factor ANOVA was used to analyze the effect of BH4 and dox on DA metabolism; when interaction between the two factors was significant, differences between groups were further estimated by using unpaired two-tailed t tests. One-factor ANOVA followed by Fisher probable least-squares difference post hoc was used to compare numbers of TH+ cells within groups over time; two-tailed unpaired t tests were used to compare numbers of TH+ cells between untreated animals and off-on or on-off-on animals, respectively, at single time-points. All values are expressed as means ± SEM. In all analyses, P < 0.05 was considered significant.
Results
Optimization of the Injection Procedure into the 6-OHDA-Lesioned Rat Striatum.
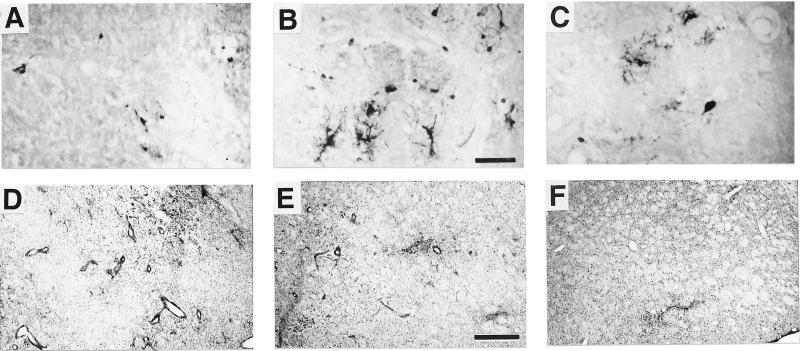
We first determined the adenoviral dose giving the optimal level of expression but limiting tissue damage (experiment a). Good dispersion of the infection throughout the striatum was ensured by delivering the virus to nine subsites as described (5). Three different doses of AdPGK⋅tet⋅hTH1, corresponding to (i) 1.75 × 108, (ii) 0.87 × 108, and (iii) 0.58 × 108 plaque-forming units, were injected into the rat striatum. In animals injected with the dose i, only a few cells were labeled with anti-TH antibodies (TH+) 10 days after surgery (Fig. 1A). On adjacent sections stained with neutral red, a clear cytolytic effect was apparent. Several enlarged blood vessels containing large numbers of perivascular cells also were observed (Fig. 1D). In contrast, the dose ii resulted in extensive TH-immunolabeling within well preserved cells of diverse morphology (Fig. 1B) throughout the striatum. Similarly, clearly defined healthy TH+ cells were observed after dose iii. However, they were fewer than with the dose ii and less dispersed (Fig. 1C). Tissue damage and perivascular cuffing were low after dose ii and almost undetectable for dose iii (Fig. 1 E and F). Thus, dose ii appeared to be suitable for effective gene transfer. In subsequent experiments, rats were given an acute immunosuppressive treatment (cyclosporin) to favor persistence of TH expression.
Figure 1.
Effects of direct injection of various doses of AdPGK⋅tet⋅hTH1 on striatal TH expression and tissue aspect. Shown are anti-TH-immunohistochemistry (A–C) and neutral red staining (D–F) of adjacent sections. (A and D) Dose i. (B and E) Dose ii. (C and F) iii. [Bar = 100 μm (A–C) and 500 μm (D–F).]
Efficacy, Persistence, and Cellular Specificity of AdPGK⋅tet⋅hTH1-Mediated TH Expression in the 6-OHDA-Lesioned Rat Striatum.
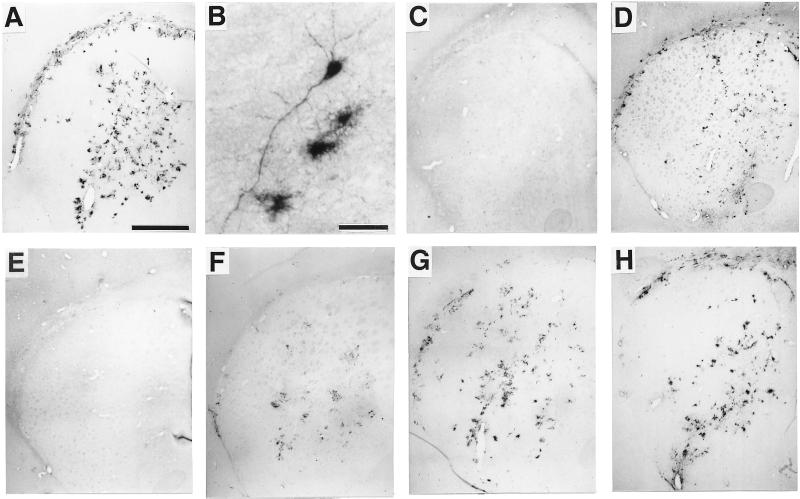
Transgene expression was evaluated after intrastriatal inoculation of AdPGK⋅tet⋅hTH1. At early time-points (experiment b), TH-immunolabeling was extensive in infected rat striata (Fig. 2 A and B): A total of ≈104 cells were TH+ (Table 1). Persistence of TH expression driven from AdPGK⋅tet⋅hTH1 was estimated in a group of animals that were killed after 17 weeks (experiment c): ≈6 × 103 cells were TH+, indicating a <50% mean drop as compared with early time-points (Fig. 2 D and H; Table 1).
Figure 2.
Striatal TH expression as visualized by anti-TH immunohistochemistry: 2.5 weeks after direct injection of AdPGK⋅tet⋅hTH1 in an untreated (A and B) or a dox-treated animal (E); 2 weeks after addition of dox to the drinking water of an on-off animal (C); 1 (F) or 3 (G) weeks after dox removal in an off-on animal; and 17 weeks after viral injection in a dox untreated (D) or in an on-off-on animal (H). [Bar = 1 mm (A and C–H) and 50 μm (B).]
Table 1.
Quantification of the total number of striatal TH+ cells at various times after injection of AdPGK⋅tet⋅hTH1
| Experiment | n | Group | Weeks after
|
TH+ cells | |
|---|---|---|---|---|---|
| Virus injection | dox +/− | ||||
| b | 4 | 2.5 | — | 9710 ± 1228 | |
| b | 3 | on | 6 | — | 9925 ± 1270 |
| c | 4 | 17 | — | 5716 ± 442* | |
| b | 7 | off | 2.5–6 | — | 0 |
| b | 3 | on-off | 4.5 | 2 | 0 |
| b | 3 | off-on | 4 | 1.5 | 3989 ± 856* |
| c | 4 | on-off-on | 17 | 10 | 4956 ± 941 |
n, number of animals; +/−, addition/removal. *, P < 0.05 vs. group on at 2.5 and 6 weeks after virus injection.
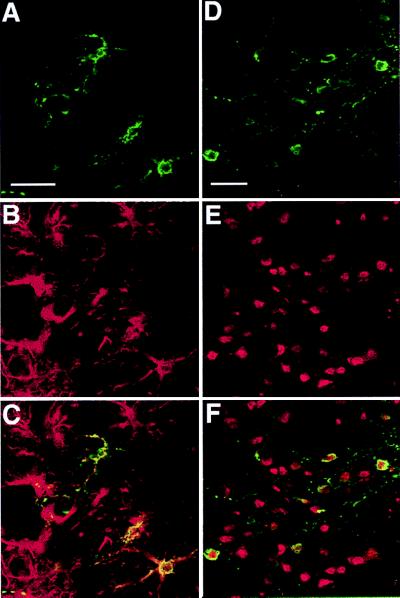
Morphological analysis of the TH+ cells suggested that the majority of them were of glial origin, although some neuron-like cells were also TH+. Double fluorescence labeling for detection of cell-specific markers and TH indicated that astrocytes (Fig. 3 A–C), neurons (Fig. 3 D–F), microglia, and oligodendrocytes (not shown) produced TH. TH+ cells expressing the astrocytic marker GFAP or the neuronal marker NeuN were counted: ≈40% of them (41.8 ± 5.6) were astrocytes and 15% (13.7 ± 2.8) neurons.
Figure 3.
Laser confocal scanned images illustrating colocalization of TH with the astrocyte marker GFAP and the neuronal marker NeuN after intrastriatal injection of AdPGK⋅tet⋅hTH1. Double-immunofluorescence of representative sections treated with anti-TH (green; A and D) and anti-GFAP (red; B) or anti-NeuN (red; E) antibodies and corresponding superposed images (C and F). (Bar = 30 μm.)
Regulated hTH1 Expression in the 6-OHDA-Lesioned Rat Striatum.
The possibility of regulating expression of TH from AdPGK⋅tet⋅hTH1 in vivo was assessed. Rats were injected with the virus and either were treated daily with dox or were not treated (experiment b). Two-and-a-half weeks after virus injection, substantial TH immunolabeling was found in animals that had not been treated with dox (Fig. 2 A and B; Table 1). In contrast, no TH+ cells were found in rats treated with dox, indicating that the production of immunodetectable amounts of TH was efficiently inhibited (Fig. 2E; Table 1). Radioactive in situ hybridization using an oligonucleotide hybridizing to the hTH mRNA confirmed these results at the transcriptional level (Fig. 5; additional information is published as supplemental data on the PNAS web site, www.pnas.org).
We tested whether TH expression could be turned off in animals already expressing the transgene and back on in animals treated with dox. Two-and-a-half weeks after virus injection, previously untreated animals were given dox (group on-off) and dox-treated animals were switched to water (group off-on). One week after addition of the antibiotic, TH immunolabeling was dramatically reduced in the striatum of on-off animals (not shown). After 2 weeks, it was abolished (Fig. 2C; Table 1). In off-on animals, TH-staining was progressively restored (Fig. 2 F and G), with numerous TH+ cells appearing as early as 1 week after removal of dox (the first time point analyzed); after 10 days, the number of TH+ cells was ≈40% of that found in animals that had not been treated with dox (Table 1).
To determine whether transgene expression could be switched repeatedly, a group of animals injected with AdPGK⋅tet⋅hTH1 (experiment c, group on-off-on) was at first not treated for 2.5 weeks. Extensive TH+ immunolabeling comparable to that obtained in experiment b was observed (not shown). Animals then were shifted for 4 weeks to dox. This resulted in abolition of TH immunolabeling (not shown). Subsequent withdrawal of dox for 10 weeks allowed restoration of TH expression (Fig. 2H) with a number of TH+ cells similar to that in animals that had never received dox (Fig. 2D, Table 1).
Apomorphine-Induced Rotational Behavior of 6-OHDA-Lesioned Rats.
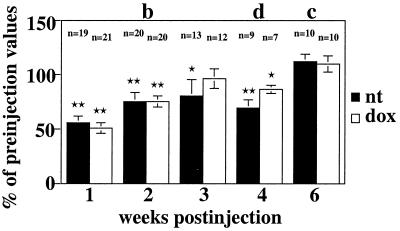
The functional effect of the AdPGK⋅tet⋅hTH1-mediated hTH synthesis in the DA-depleted striatum and its dependence on treatment with dox were evaluated. Apomorphine-induced rotations were quantified at various time-points after inoculation of the virus and were compared with preinjection values. A significant overall reduction of apomorphine-induced rotation was observed early after injection of the virus (Fig. 4). However, after 6 weeks, pre- and postinjection measurements were similar. There was no significant difference between values obtained in untreated and dox-treated animals at any time-point.
Figure 4.
Quantification of apomorphine-induced rotation in untreated (nt) and dox-treated animals at various times after injection of AdPGK⋅tet⋅hTH1 (experiments b–d). Asterisks indicate significant differences between pre- and postinjection rotational scores within groups (P ≤ 0.01 for double and ≤ 0.05 for single asterisks). n, number of animals.
AdPGK⋅tet⋅hTH1-Mediated Production of dopa, DA, and its Metabolites Diydroxyphenylacetic Acid and HVA in the 6-OHDA-Lesioned Rat Striatum.
After inoculation of AdPGK⋅tet⋅hTH1, rats were either untreated or treated with dox for 4 weeks and were killed (experiment d). Striata were dissected, and dopa, DA, diydroxyphenylacetic acid, and HVA were assayed in the lesioned and unlesioned striatum of each animal. Virus-injected animals that had been allowed to express the transgene displayed similar tissue levels of dopa, DA, and its metabolites as virus-injected dox-treated animals (Table 2).
Table 2.
Effect of AdPGK⋅tet⋅hTH1 on levels of dopa, DA, and its metabolites in the 6-OHDA-lesioned rat striatum
| n | dox | BH4 | Percent of values in corresponding contralateral striata
|
|||
|---|---|---|---|---|---|---|
| Dopa | DA | DOPAC | HVA | |||
| 7 | − | 37.7 ± 6.5 | 2.8 ± 0.6 | 3.8 ± 1.4 | 6.0 ± 2.2 | |
| No | ||||||
| 6 | + | 24.3 ± 10.8 | 2.6 ± 1.0 | 3.9 ± 1.6 | 4.1 ± 2.3 | |
| 10 | − | 466.4 ± 9.1* | 1.1 ± 0.4 | 6.1 ± 1.2 | 18.5 ± 4.3† | |
| Yes | ||||||
| 9 | + | 57.7 ± 16.5 | 0.8 ± 0.3 | 3.5 ± 0.5 | 2.5 ± 1.8 | |
DOPAC, diydroxyphenylacetic acid; n, number of animals.
*P ≤ 0.01 and †P < 0.05 vs. all other groups.
We evaluated whether exogenous administration of the TH cofactor BH4 caused an increase in DA metabolism in the AdPGK⋅tet⋅hTH1-injected lesioned striatum. Some of the animals in experiment d received a subcutaneous injection of BH4 before sacrifice, a treatment that significantly increases brain levels of BH4 (27). In animals that had been allowed to express the transgene, contents of dopa in lesioned striata were significantly higher than in all other groups, representing ≈460% of contents in corresponding contralateral striata (Table 2). Levels of the DA metabolite HVA were also significantly increased, reaching ≈20% of those measured in contralateral striata. In contrast, no stimulating effect of BH4 was observed in virus-injected animals treated with dox.
Discussion
We analyzed the effect of the direct injection of AdPGK⋅tet⋅hTH1 (22), an adenovirus encoding hTH1 under the control of the tet-off regulatory system, into the DA-depleted striatum of 6-OHDA-lesioned rats. We demonstrated the efficacy of this virus for the controlled delivery of a transgene with therapeutic potential into the brain. In the appropriate experimental conditions, AdPGK⋅tet⋅hTH1 allowed efficient delivery of the TH transgene to the whole striatum. TH immunolabeling was detected in a large number of striatal cells of glial and neuronal type and was persistent. Most importantly, dox allowed efficient reversible control of transgene expression.
Exogenous control of the synthesis of the therapeutic gene product has become a major requirement for gene therapy. Several attempts are being made to construct vectors for the direct controlled delivery of gene products to the central nervous system. Gene regulatory cassettes have been inserted into herpes simplex virus-based vectors, adeno-associated virus vectors, and adenoviral vectors to control the expression of reporter genes in the brain (28–30). One particularly advanced study reported the development of a two vector-based tetracycline-regulatable adenoviral system for on-off switching of a transgene encoding enhanced green fluorescent protein (30). The design of a gene delivery system to incorporate into one DNA molecule both the regulatory system and the transgene would be an important step toward effective and safe clinical application. AdPGK⋅tet⋅hTH1 is an adenovirus vector that meets this criterion. It allowed efficient and repeated on-off switching of TH after direct intrastriatal injection: TH immunolabeling in the rat striatum was abolished within 2 weeks of inclusion of dox in the animal’s drinking water; conversely, after withdrawal of dox, large numbers of TH+ cells appeared within 1 week, and immunostaining was progressively restored. Interestingly, resumption of TH expression was considerably more delayed in intrastriatal grafts of human neural progenitor cells infected ex vivo with AdPGK⋅tet⋅hTH1, suggesting a slower clearance rate of dox from human transplants than from rat striatal tissue (22). In general, the delay required for gene reactivation could be shortened by the use of lower doses of dox that may still be sufficient to inhibit transgene expression (22).
Another major requirement for effective gene therapy is the maintenance of gene expression. One factor that may compromise the duration of adenovirus-mediated gene expression is a strong cytotoxicity. Accurate adjustment of the viral dose can obviate this detrimental effect: The optimal dose allowed expression of the transgene in a large number of cells whereas double this dose resulted in limited expression and a disrupted striatal tissue, clearly indicative of a cytopathic effect. Another factor that could limit the efficacy of gene transfer mediated by first generation recombinant adenoviruses is the host immune response to infected cells. Although less effective than in peripheral organs, the inflammatory reaction elicited by these vectors does also exist in the central nervous system, where it is accompanied by a marked decline in the number of transduced cells over time (31, 32). Immunosuppressing drugs interfering with the proliferation and differentiation of T-cells may reduce T-cell infiltration into the injection site and facilitate maintenance of gene expression (33, 34). We therefore combined the use of the appropriate viral dose with transient immunosuppression with cyclosporin. In these conditions, transgene expression in the rat striatum was maintained for at least 17 weeks with an only moderate decrease in the number of TH+ cells. Despite the immunosuppressive treatment, however, a host inflammatory reaction was observed in injected striata that was particularly important early after virus inoculation. Indeed, 2.5 weeks after injection of AdPGK⋅tet⋅hTH1, numerous CD4+ and CD8+ cells clustered at the injection site. At later time-points, inflammation was less important: Although CD4+ cells persisted throughout the injected striatum until 17 weeks, they were considerably fewer than at early time-points and more dispersed; only a few CD8+ cells were detected at 6 weeks but no more at later time-points (Fig. 6; see supplemental data at www.pnas.org).
Apomorphine-induced rotational asymmetry in 6-OHDA lesioned rats is a behavioral indicator of the extent of DA restoration in the denervated striatum (35, 36). We have previously reported significant behavioral recovery in this animal model 1 and 2 weeks after the intrastriatal injection of an adenovirus encoding hTH1; in contrast, control animals that had received a virus encoding a lacZ reporter gene did not modify their behavior (5). However, the mean decrease of apomorphine-induced rotation was only modest (30% after 1 week, 22% after 2 weeks) as compared with that observed after transplantation of a dopa-producing cell line (50%) (23). Here, AdPGK⋅tet⋅hTH1 allowed reliable analysis of the behavioral data obtained after the direct TH gene transfer to the 6-OHDA lesioned striatum. Driving synthesis of TH in a conditional manner, this vector provides a good internal control, eliminating experimental bias related to the use of different vectors with independent characteristics concerning their stock, titer, dilution volume, etc. As previously observed, there was a general decrease in the apomorphine-induced rotation shortly after injection of AdPGK⋅tet⋅hTH1. However, this decrease was only transient: The rotational scores increased progressively and were similar to preinjection values 6 weeks after infection, a time at which TH immunolabeling in the infected lesioned striata was as widespread as earlier. Most importantly, there was no difference between values obtained for animals that had been treated or not treated with dox, demonstrating that the observed short-term behavioral recovery was not correlated with the expression of TH. This latter phenomenon may rather be a consequence of the strong early virus-induced inflammatory response (Fig. 6; see supplemental data).
In accordance with the lack of functional effect associated with the intrastriatal expression of hTH1, the striatal contents of dopa, DA, and its metabolites were no higher in animals injected with AdPGK⋅tet⋅hTH1 than in 6-OHDA-lesioned controls. Presumably, transgenic hTH was not active in the lesioned host striatum. The hydroxylation of tyrosine mediated by TH requires the cofactor BH4 (37). Levels of BH4 are substantially lowered in PD patients (38, 39) and in the 6-OHDA denervated striatum (40), such that residual BH4 may be insufficient to promote significant exogenous TH activity. This hypothesis is supported by the finding that a subcutaneous injection of BH4 induced a substantial increase of tissue dopa levels in lesioned striata of rats that had received AdPGK⋅tet⋅hTH1. Tissue levels of HVA were also significantly increased, indicating that, in these conditions, DA was synthesized and subsequently degraded. In a previous study, high striatal tissue levels of dopa obtained after grafting of a cell line genetically modified to express TH1 were accompanied by a significant increase of extracellular DA and resulted in behavioral recovery in 6-OHDA-lesioned rats (23).
Several other studies have underlined the essential role of BH4 in experiments involving the transfer of a TH transgene into animal models of PD. The first evidence was from ex vivo approaches involving the use of primary fibroblasts genetically modified to synthesize TH (41–43). These cells lack a GTP-cyclohydrolase I activity catalyzing the rate-limiting step in the biosynthesis of BH4. After grafting to the 6-OHDA-lesioned striatum, synthesis of dopa was promoted only if the cells were engineered to coexpress GTP-cyclohydrolase I or after exogenous BH4 infusions. Recently, it has been shown that the conversion of part of the striatal cell population to TH-synthesizing cells by direct infection using an adeno-associated virus vector also requires the delivery of BH4 to allow production of dopa (44).
Both our results and this latter study are inconsistent with two other previous investigations reporting the long-term functional efficacy of a therapy based on the direct adeno-associated virus or herpes simplex virus vector-mediated transfer of a TH cDNA to the striatum of 6-OHDA-lesioned rats (6, 7). However, in the first case, evaluation of DA metabolism was not reported (7), and, in the second case, transduction efficiency was extremely low (5–300 total TH+ cells) (6), raising concerns about the correlation between the observed phenotypic correction and the intrastriatal production of exogenous TH (44, 45). In light of our results and the above discussed studies, it appears that successful gene replacement paradigms aimed at reverting the dopaminergic deficit of PD will have to include concomitant transfer of TH and GTP-cyclohydrolase I transgenes. Other factors involved in the biosynthesis of DA may be necessary to optimize this approach, i.e., the enzyme aromatic l-amino acid decarboxylase promoting the conversion of dopa to DA.
In conclusion, we demonstrate that the combination of a tetracycline-sensitive gene regulatory cassette with a single adenovirus vector is a powerful system for the obtention of efficient, sustained, and controlled synthesis of a gene product in the rodent brain. Transposed to new generations of adenovirus vectors optimized for minimal cytotoxicity and immunogenicity, this system should provide a substantial advance toward the development of beneficial and safe therapies for human neurodegenerative diseases.
Supplementary Material
Acknowledgments
We thank P. Horellou and G. Pitiot for helpful discussions and critical review of the manuscript. We thank Novartis for providing cyclosporin. This work was supported by the Association Française contre les Myopathies, the Centre National de la Recherche Scientifique, the Conseil Régional d’Ile de France, European Community Contract 951012, the Institut pour la Recherche sur la Moelle Epinière, and Retina France.
Abbreviations
- PD
Parkinson’s disease
- DA
dopamine
- TH
tyrosine hydroxylase
- hTH1
human tyrosine hydroxylase 1
- dox
doxycycline
- GFAP
glial fibrillary acidic protein
- HVA
homovanillic acid
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Gerlach M, Riederer P. In: Pharmacology and Clinical Use in Neurodegenerative Disorders. Szelenyi I, editor. Basel: Birkhauser; 1993. pp. 25–50. [Google Scholar]
- 2.Marsden C D, Parkes J D. Lancet. 1976;i:292–296. doi: 10.1016/s0140-6736(76)91416-1. [DOI] [PubMed] [Google Scholar]
- 3.Horellou P, Bilang B A, Mallet J. Neurobiol Dis. 1997;4:280–287. doi: 10.1006/nbdi.1997.0162. [DOI] [PubMed] [Google Scholar]
- 4.Raymon H K, Thode S, Gage F H. Exp Neurol. 1997;144:82–91. doi: 10.1006/exnr.1996.6392. [DOI] [PubMed] [Google Scholar]
- 5.Horellou P, Vigne E, Castel M N, Barneoud P, Colin P, Perricaudet M, Delaere P, Mallet J. NeuroReport. 1994;6:49–53. doi: 10.1097/00001756-199412300-00014. [DOI] [PubMed] [Google Scholar]
- 6.During M J, Naegele J R, O’Malley K L, Geller A I. Science. 1994;266:1399–1403. doi: 10.1126/science.266.5189.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaplitt M G, Leone P, Samulski R J, Xiao X, Pfaff D W, O’Malley K L, During M J. Nat Genet. 1994;8:148–154. doi: 10.1038/ng1094-148. [DOI] [PubMed] [Google Scholar]
- 8.Barkats M, Bilang B A, Buc C M, Castel B M, Corti O, Finiels F, Horellou P, Revah F, Sabate O, Mallet J. Prog Neurobiol. 1998;55:333–341. doi: 10.1016/s0301-0082(98)00028-8. [DOI] [PubMed] [Google Scholar]
- 9.Mitani K, Graham F L, Caskey C T, Kochanek S. Proc Natl Acad Sci USA. 1995;92:3854–3858. doi: 10.1073/pnas.92.9.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amalfitano A, Hauser M A, Hu H, Serra D, Begy C R, Chamberlain J S. J Virol. 1998;72:926–933. doi: 10.1128/jvi.72.2.926-933.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schiedner G, Morral N, Parks R J, Wu Y, Koopmans S C, Langston C, Graham F L, Beaudet A L, Kochanek S. Nat Genet. 1998;18:180–183. doi: 10.1038/ng0298-180. [DOI] [PubMed] [Google Scholar]
- 12.Burcin M M, Schiedner G, Kochanek S, Tsai S Y, O’Malley B W. Proc Natl Acad Sci USA. 1999;96:355–360. doi: 10.1073/pnas.96.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harvey D M, Caskey C T. Curr Opin Chem Biol. 1998;2:512–518. doi: 10.1016/s1367-5931(98)80128-2. [DOI] [PubMed] [Google Scholar]
- 14.Clackson T. Curr Opin Chem Biol. 1997;1:210–218. doi: 10.1016/s1367-5931(97)80012-9. [DOI] [PubMed] [Google Scholar]
- 15.Gossen M, Bujard H. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gossen M, Freundlieb S, Bender G, Muller G, Hillen W, Bujard H. Science. 1995;268:1766–1769. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]
- 17.Saez E, No D, West A, Evans R M. Curr Opin Biotechnol. 1997;8:608–616. doi: 10.1016/s0958-1669(97)80037-7. [DOI] [PubMed] [Google Scholar]
- 18.Chen J, Kelz M B, Zeng G, Sakai N, Steffen C, Shockett P E, Picciotto M R, Duman R S, Nestler E J. Mol Pharmacol. 1998;54:495–503. doi: 10.1124/mol.54.3.495. [DOI] [PubMed] [Google Scholar]
- 19.Mayford M, Bach M E, Huang Y Y, Wang L, Hawkins R D, Kandel E R. Science. 1996;274:1678–1683. doi: 10.1126/science.274.5293.1678. [DOI] [PubMed] [Google Scholar]
- 20.Yu J S, Sena E M, Paulus W, Breakefield X O, Reeves S A. Cancer Res. 1996;56:5423–5427. [PubMed] [Google Scholar]
- 21.Corti O, Horellou P, Colin P, Cattaneo E, Mallet J. NeuroReport. 1996;7:1655–1659. doi: 10.1097/00001756-199607080-00026. [DOI] [PubMed] [Google Scholar]
- 22.Corti O, Sabate O, Horellou P, Colin P, Dumas S, Buchet D, Buc C M, Mallet J. Nat Biotechnol. 1999;17:349–354. doi: 10.1038/7901. [DOI] [PubMed] [Google Scholar]
- 23.Horellou P, Brundin P, Kalen P, Mallet J, Björklund A. Neuron. 1990;5:393–402. doi: 10.1016/0896-6273(90)90078-t. [DOI] [PubMed] [Google Scholar]
- 24.Hudson J L, van Horne C G, Stromberg I, Brock S, Clayton J, Masserano J, Hoffer B J, Gerhardt G A. Brain Res. 1993;626:167–174. doi: 10.1016/0006-8993(93)90576-9. [DOI] [PubMed] [Google Scholar]
- 25.Abercrombie M. Anat Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- 26.Adrien J, Lanfumey L, Gozlan H, Fattaccini C M, Hamon M. J Pharmacol Exp Ther. 1989;248:1222–1230. [PubMed] [Google Scholar]
- 27.Brand M P, Hyland K, Engle T, Smith I, Heales S J. J Neurochem. 1996;66:1150–1156. doi: 10.1046/j.1471-4159.1996.66031150.x. [DOI] [PubMed] [Google Scholar]
- 28.Ho D Y, McLaughlin J R, Sapolsky R M. Brain Res Mol Brain Res. 1996;41:200–209. doi: 10.1016/0169-328x(96)00097-6. [DOI] [PubMed] [Google Scholar]
- 29.Haberman R P, McCown T J, Samulski R J. Gene Ther. 1998;5:1604–1611. doi: 10.1038/sj.gt.3300782. [DOI] [PubMed] [Google Scholar]
- 30.Harding T C, Geddes B J, Murphy D, Knight D, Uney J B. Nat Biotechnol. 1998;16:553–555. doi: 10.1038/nbt0698-553. [DOI] [PubMed] [Google Scholar]
- 31.Wood M J, Charlton H M, Wood K J, Kajiwara K, Byrnes A P. Trends Neurosci. 1996;19:497–501. doi: 10.1016/S0166-2236(96)10060-6. [DOI] [PubMed] [Google Scholar]
- 32.Kajiwara K, Byrnes A P, Charlton H M, Wood M J, Wood K J. Hum Gene Ther. 1997;8:253–265. doi: 10.1089/hum.1997.8.3-253. [DOI] [PubMed] [Google Scholar]
- 33.Durham H D, Alonso V M, Sadikot A F, Zhu L, Lochmüller H, Massie B, Nalbantoglu J, Karpati G. NeuroReport. 1997;8:2111–2115. doi: 10.1097/00001756-199707070-00005. [DOI] [PubMed] [Google Scholar]
- 34.Geddes B J, Harding T C, Hughes D S, Byrnes A P, Lightman S L, Conde G, Uney J B. Endocrinology. 1996;137:5166–5169. doi: 10.1210/endo.137.11.8895393. [DOI] [PubMed] [Google Scholar]
- 35.Ungerstedt U, Arbuthnott G W. Brain Res. 1970;24:485–483. doi: 10.1016/0006-8993(70)90187-3. [DOI] [PubMed] [Google Scholar]
- 36.Freed W J, Ko G N, Niehoff D L, Kuhar M J, Hoffer B J, Olson L, Cannon S H, Morihisa J M, Wyatt R J. Science. 1983;222:937–939. doi: 10.1126/science.6635666. [DOI] [PubMed] [Google Scholar]
- 37.Nagatsu T. Essays Biochem. 1995;30:15–35. [PubMed] [Google Scholar]
- 38.Nagatsu T, Yamaguchi T, Kato T, Sugimoto T, Matsuura S, Akino M, Nagatsu I, Iizuka R, Narabayashi H. Clin Chim Acta. 1981;109:305–311. doi: 10.1016/0009-8981(81)90316-8. [DOI] [PubMed] [Google Scholar]
- 39.LeWitt P A, Miller L P, Newman R P, Burns R S, Insel T, Levine R A, Lovenberg W, Calne D B. Adv Neurol. 1984;40:459–462. [PubMed] [Google Scholar]
- 40.Levine R A, Miller L P, Lovenberg W. Science. 1981;214:919–921. doi: 10.1126/science.6117945. [DOI] [PubMed] [Google Scholar]
- 41.Uchida K, Tsuzaki N, Nagatsu T, Kohsaka S. Dev Neurosci (Basel) 1992;14:173–180. doi: 10.1159/000111661. [DOI] [PubMed] [Google Scholar]
- 42.Leff S E, Rendahl K G, Spratt S K, Kang U J, Mandel R J. Exp Neurol. 1998;151:249–264. doi: 10.1006/exnr.1998.6803. [DOI] [PubMed] [Google Scholar]
- 43.Bencsics C, Wachtel S R, Milstien S, Hatakeyama K, Becker J B, Kang U J. J Neurosci. 1996;16:4449–4456. doi: 10.1523/JNEUROSCI.16-14-04449.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mandel R J, Rendahl K G, Spratt S K, Snyder R O, Cohen L K, Leff S E. J Neurosci. 1998;18:4271–4284. doi: 10.1523/JNEUROSCI.18-11-04271.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Isacson O. Science. 1995;269:856–857. doi: 10.1126/science.7638605. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.