Abstract
The waggler, a neurological mutant mouse with a disrupted putative neuronal Ca2+ channel γ subunit, exhibits a cerebellar granule cell-specific brain-derived neurotrophic factor deficit, severe ataxia, and impaired eyeblink conditioning. Here, we show that multiple synapses of waggler cerebellar granule cells are arrested at an immature stage during development. Synaptic transmission is reduced at parallel fiber–Purkinje cell synapses. The Golgi cell–granule cell synaptic currents show immature kinetics associated with reduced γ-aminobutyric acid type A receptor α6 subunit expression in granule cells. In addition, the mossy fiber–granule cell synapses exhibit N-methyl-d-aspartate (NMDA) receptor-mediated excitatory postsynaptic currents (EPSCs), but not α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor-mediated EPSCs. Our results suggest that voltage-dependent Ca2+ channels are involved in synapse maturation. This deficient synaptic transmission in the waggler cerebellum may account for their behavioral deficits.
The waggler (stgwag) is a recessive neurological mutation on chromosome 15 that arose spontaneously in the MRL/MpJ mouse strain. We have previously reported (1) that homozygous wagglers (later referred to as wagglers) exhibit normal cerebellar foliation and laminar structures. The cerebellar granule cells migrate completely into internal granule cell layer and survive normally in adult mice. However, waggler cerebellar granule cells do not exhibit developmental up-regulation of brain-derived neurotrophic factor (BDNF) expression, which normally occurs after granule cell maturation (2, 3). Behaviorally, wagglers fail to develop normal gait and motor coordination, and adult wagglers are severely impaired in cerebellum-dependent eyeblink conditioning (1). It appears that the waggler mutation may disrupt the functional integrity of the cerebellar circuitry.
The Ca2+ channel γ subunit was discovered in skeletal muscle (4, 5), and was found to modulate calcium currents in a variety of ways (6, 7). Recently, the gene (Cacng2) that is mutated in wagglers was cloned and found to encode a putative neuronal calcium channel γ subunit—stargazin (8). Expression of stargazin in a cell line expressing neuronal α1 class A Ca2+ channels (including α1A/β1A/α2/δ) shifted steady-state inactivation toward hyperpolarization, as has also been shown for the muscle Ca2+ channel γ subunit (6). Cacng2 is ubiquitously expressed in the brain, with cerebellar cortex among the regions of highest expression (8). The distribution of Cacng2 mRNA signals in the cerebellum is consistent with granule cell layer demarcation. The waggler mutation results in a premature termination of the Cacng2 transcript, leading to a substantially lower level of stargazin in the mutant mice (8). One potential effect of such reduction in stargazin is abnormal Ca2+ entry through voltage-dependent calcium channels (VDCCs) in waggler granule cells. Previous studies have shown that neuronal discharges (9) and subsequent activation of VDCCs (10) regulate the developmental transition of γ-aminobutyric acid-A (GABAA) receptor subunit expressions, a landmark of granule cell maturation (11, 12). In addition, chronic depolarization-mediated Ca2+ entry also differentially regulates expression of N-methyl-d-aspartate (NMDA) receptors and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (13–16), which normally occurs during granule cell maturation (17–19). Thus, it is possible that the waggler mutation impairs cerebellar granule cell maturation and consequently causes the behavioral deficits. In the present study, we examined the maturation of cerebellar glutamatergic mossy fiber (mf)-granule cell synapses, GABAergic Golgi cell–granule cell synapses, and glutamatergic parallel fiber–Purkinje cell (pf-PC) synapses in waggler mutant mice.
Materials and Methods
Animals.
Heterozygous waggler mice (on C57BL/6 genetic background) were originally obtained from The Jackson Laboratory and were used for breeding in a University of Southern California vivarium. Homozygous mutant mice were identified by the ataxic gait. Heterozygous and homozygous wild-type littermates were used in the experiments as controls.
Histology.
Adult mutant and wild-type mice at postnatal day 28 (P28) were deeply anesthetized with halothane and transcardiacally perfused with 0.9% saline and 10% buffered formalin. The brains were removed and postfixed in 10% formalin for at least 24 hr. For general histological examination, the brain was sliced with a sliding microtome. Coronal and parasagittal sections (40 μm) were mounted onto gelatin-coated slides, stained with cresyl violet, and observed with a light microscope. For Golgi staining, the cerebellum was cut into 2-mm3 cubes, and further fixed with 0.33% OsO4 and 2.6% K2Cr2O7 for 6 days. Then, the brain tissues were stained with 0.75% AgNO3 for 20 hr. After being rinsed with ethanol, the tissues were sliced (100 μm) with a Vibratome, and the sections treated with xylene for 1 hr, mounted with Permount, and observed with a light microscope. To assess the cell morphology, 20 granule cells with clearly identified somas, dendritic branches, and glomeruli were sampled for each phenotype. Two-dimensional images were used to measure the somatic and dendritic sizes. The long diameter was defined as the largest distance between two points on the somatic periphery. The short diameter was defined as the longest diameter perpendicular to the long axis. The dendritic length was measured from somatic periphery to the glomerulus. Granule cell count was performed as described previously (20). Briefly, fixed tissues were dehydrated, infiltrated with propylene oxide, embedded in Epon (which was polymerized overnight at 67°C), and cut into semithin sections (3 μm). The sections were deplasticized in NaOH/ethanol solution and stained with hematoxylin and eosin. Numbers of granule cells were counted in 6500-μm 2 rectangular areas located in lobules 6 and 8. Cells on the left and bottom edges were counted, and those on top and right edges were not.
In Situ Hybridization.
The mutant and wild-type (P28–P35) brain sections (16 μm, fresh frozen) were mounted on the same slides and processed identically for optimal comparison. The sections were fixed with 4% paraformaldehyde in 0.1 M PBS and pretreated with 0.25% acetic anhydride and 0.1 M triethanolamine. Then, the tissue sections were incubated overnight at 50°C with 35S-labeled oligonucleotide probes [50% formamide, 10% dextran sulfate, 300 mM NaCl, 0.5 mg/ml yeast RNA, 10 μM DTT, 0.02% Ficoll, 0.02% polyvinyl pyrrolidone, 0.02% BSA, 1 mM EDTA, and 10 mM Tris⋅HCl (pH 8.0)]. After hybridization, the sections were rinsed, dried, and autoradiographed. Probe sequences for pam GABAA receptor α6 mRNAs (21), flip and flop forms of GluR1–3 (22) and GluR4 (23) were adopted from previous studies.
Electrophysiology.
Cerebellar sagittal slices (400 μm) were prepared following standard procedures and incubated in artificial cerebrospinal fluid (ACSF; 124 mM NaCl/3 mM KCl/1.25 mM KH2PO4/3 mM CaCl2/1 mM MgCl2/26 mM NaHCO3/10 mM glucose, buffered with 5% CO2/95% O2) for at least 30 min. Incubations and all subsequent experiments were performed at room temperature. Cerebellar pf-PC excitatory postsynaptic potentials (EPSPs) were recorded with sharp electrodes (80–120 MΩ, filled with 2 M potassium-acetate) in the presence of picrotoxin (40 μM). Purkinje cells were current-clamped at a resting membrane potential of −80 mV. To study paired-pulse facilitation, biphasic electrical stimulation of the outer molecular layer was adjusted to elicit EPSPs with amplitudes of 5–7 mV. A series of paired-pulse stimuli with variable interpulse intervals was applied at a frequency of 0.1 Hz. To examine input–output relations of the pf-PC EPSPs, cell membrane potentials were current-clamped at −80 to −90 mV, and a series of stimuli (5–50 μA, 5 μA steps) was applied at 0.1 Hz. Serial resistance and input resistance were frequently monitored throughout the experiment.
Granule cell membrane currents were recorded with whole-cell configuration, and the “blind” techniques (24) were used to form tight seals. The impedance of the patch pipettes ranges from 3 to 6 MΩ when filled with internal solutions [whole-cell solution for recording GABAA receptor currents: 140 mM CsCl/2 mM MgCl2/5 mM EGTA/10 mM Hepes/4 mM Na2ATP/0.3 mM Na3GTP (pH 7.3); for recording AMPA receptor or NMDA receptor currents: 108 mM CsMeSO4/4.5 mM MgCl2/9 mM EGTA/4 mM MgATP/0.3 mM Na3GTP/24 mM Hepes (pH 7.3)]. Granule cells were identified by their typical low whole-cell capacitance (<5 pF) and high input resistance (>1 GΩ) (17). To record evoked EPSCs at the mf-granule cell synapses, picrotoxin (40 μM) was included in ACSF. Biphasic stimulation (0.1 Hz) was delivered through a coaxial electrode placed on nearby white matter. AMPA receptor EPSCs were recorded in the presence of d-2-amino-5-phosphonovaleric acid (100 μM) with Vm at −80 mV. NMDA receptor EPSCs were measured with 6-cyano-7-nitroquinoxaline (CNQX; 10 μM) in ACSF and Vm = +60 mV. To record AMPA-activated whole-cell currents, picrotoxin (40 μM) was included to block spontaneous GABAA receptor currents, and tetrodotoxin (0.5 μM) to prevent trans-synaptic activation of granule cell synapses. AMPA (500 μM) was applied [20 psi, 1 s (1 psi = 6.9 kPa)] through a picospritzer (General Valve, Fairfield, NJ) into the perfusion ACSF in the close vicinity of the recorded granule cells. Pclamp6 programs (Axon Instruments, Foster City, CA) were used for data acquisition and the analysis of evoked responses. To study GABAA receptor-mediated Golgi cell–granule cell synaptic currents, kynurenic acid (2 mM) was applied extracellularly to eliminate glutamatergic synaptic currents. Spontaneous GABAA synaptic currents were continuously recorded (Vm = −80 mV) for 1 min. Spontaneous GABAA receptor-mediated events were identified with custom-made programs and were later visually verified by experimenters. Decay time constant of the GABAA receptor-mediated synaptic currents was calculated by using the average of all events in the 1-min recording period. All error bars in the figures indicate SEM.
BDNF Treatment.
Brain slices (both waggler and WT, P17, prepared as described above) were incubated in ACSF containing 100 ng/ml BDNF (human recombinant; Sigma) at room temperature for 12 hr (25). A set of WT slices were also incubated under the same condition, except that no BDNF was used to assess the effects of BDNF on wild-type granule cells. After incubation, slices were transferred to a submission recording chamber and recorded as described before. During the recordings, slices were perfused with normal ACSF without BDNF.
Results
Normal Cerebellar Architecture, but Reduced Cerebellar Granule Cell Sizes in waggler Mutant Mice.
The adult waggler cerebellum exhibits normal foliation (Fig. 1A) and laminar structure. The thickness of each layer is comparable between mutants and age-matched wild types. In adult wagglers, all granule cells finish migration and are located in the internal granule cell layer. We examined granule cell density in the central vermal areas of lobules 6 and 8, and found a slight, but nonsignificant, increase in granule cell density in wagglers (Fig. 1B; n = 12 for each group). Adult mutant granule cells exhibited claw-like telodendria (Fig. 1C), characteristic of differentiated granule cells. Quantification of granule cell sizes indicated that mutant granule cells exhibited smaller short somatic diameters (Fig. 1D; n = 20 for each group), suggesting an irregular shape of the soma. Dendritic length was also reduced in waggler granule cells (Fig. 1D; n = 70 for WT, and n = 58 for wag). These results indicate that waggler external germinal cells proliferate, migrate into internal granule cell layer, and differentiate into granule cells, which survive with normal gross morphology.
Figure 1.
Cerebellar histology and granule cell morphology. (A) Midsagittal sections of the cerebellum stained with cresyl violet. The waggler cerebellum exhibits laminar structure. (B) Cerebellar granule cell density. (C) Golgi staining of cerebellar granule cells. waggler granule cells show characteristic morphology. (Scale bar: 10 μm.) (D) Granule cell sizes. The short somal axis and dendritic length are significantly reduced in wagglers (∗, P < 0.05).
Altered pf-PC Synaptic Transmission in wagglers.
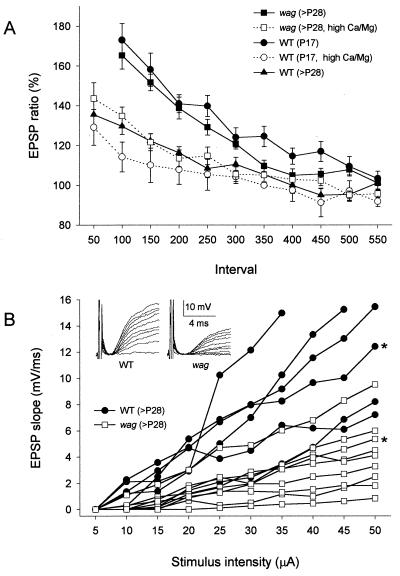
During early postnatal development, as migrating granule cells pass the molecular layer, they extend axon collaterals as parallel fibers, which form synapses with expending Purkinje cell dendrites. This process occurs during the second and third postnatal weeks (26). We studied the developmental changes in pf-PC EPSPs by examining paired-pulse facilitation (PPF), a phenomenon generally believed to be due to presynaptic mechanisms (27, 28). The pf-PC synaptic responses are mediated by AMPA receptor in wagglers, as well as in wild types, and were completely blocked by 10 μM CNQX (data not shown). Compared with adult (older than postnatal 28th day, >P28) wild-type synapses, developing (P17) wild-type pf-PC synapse exhibited higher PPF of EPSP amplitudes over the range of 100- to 300-ms interpulse interval (Fig. 2A), suggesting low transmitter release probabilities (29). Raising the extracellular [Ca2+]/[Mg2+] ratio from 3 to 6 ([Ca2+] from 3 to 4.2 mM, and [Mg2+] from 1 to 0.7 mM), which increases transmitter release probability, reduced the degree of PPF at developing synapses to that of adult synapses. Interestingly, PPF of the adult mutant synapses was significantly greater than that of adult wild-type synapses, and resembled that of developing synapses. Raising extracellular [Ca2+]/[Mg2+] ratio reduced PPF of adult mutant synapses, as it did for P17 wild-type synapses. These results suggest that transmitter release probability is low at waggler granule cell terminals, possibly because of impaired synapse maturation. We also compared input–output relations in adult wild-type and mutant pf-PC synapses. The pf-PC EPSPs exhibited smaller slopes of input–output function and lower maximal responses in mutants than in wild types (Fig. 2B).
Figure 2.
Synaptic transmission at waggler pf-PC synapse is reduced. (A) PPF is significantly enhanced at young wild-type and adult waggler pf-PC synapses over the intervals ranging from 50 to 300 ms. Raising extracellular [Ca2+]/[Mg2+] from 3 to 6 reduced PPF of adult waggler and young wild-type synapses to adult wild-type level. (B) Input–output relations at adult pf-PC synapses. Each line represents responses recorded from an individual Purkinje cell. With gradually increased intensity of parallel fiber stimulation, pf-PC EPSPs exhibited smaller slopes of the input–output function and lower maximal responses in wagglers than wild types. The Insets represent typical examples of EPSP traces from both groups (marked with asterisk).
Slow Golgi Cell–Granule Cell GABAA Synaptic Currents and Reduced GABAA Receptor α6 Subunit Expression in waggler Mutants.
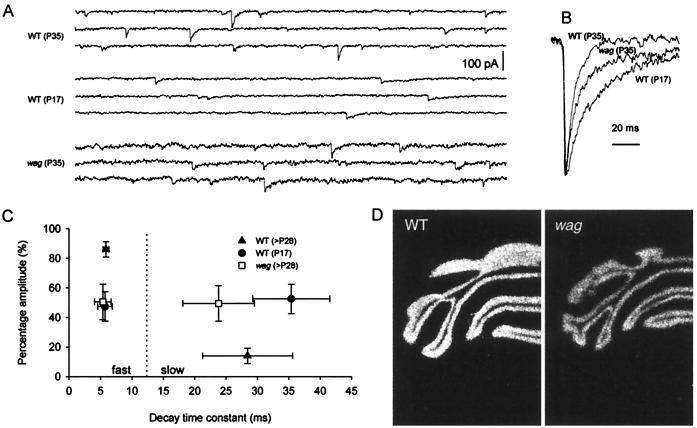
Cerebellar granule cells express different combinations of GABAA receptor at Golgi cell–granule cell synapses during development (12, 30, 31). For example, the α6 subunit of the GABAA receptors is expressed only in mature cerebellar granule cells and is considered a molecular marker of maturity (12, 32). In addition to changes in receptor subunit expression, GABAA receptor-mediated Golgi cell–granule cell synaptic currents also undergo a developmental transition from slow-decaying to fast-decaying currents (33), which may be because of the switch in GABAA receptor subunit composition (32, 33). We compared whole-cell spontaneous GABAA currents from wagglers and wild types. Excitatory current was blocked by adding kynurenic acid (2 mM) in the perfusion bath. The remaining spontaneous currents were completely blocked by the GABAA receptor antagonist picrotoxin (data not shown). Adult wild-type granule cells (n = 10) exhibited typical fast GABAA currents (Fig. 3 A–C). In contrast, the responses recorded from adult (P35) waggler granule cells (n = 6) were much slower, had an increased amount of slow component, and resembled responses from immature (P17) wild-type granule cells (n = 9). In situ hybridization revealed that GABAA receptor α6 subunit expression was reduced by 20% in adult (P28) waggler granule cells as compared with adult wild types (OD: WT, 8718 ± 120, n = 13; wag, 6942 ± 182, n = 13; P < 0.001. See Fig. 3D for examples). A similar reduction in GABAA receptor α6 subunit protein level was previously described in stargazer granule cells (34). Thus, the granule cells in the adult waggler cerebellum are not fully mature.
Figure 3.
The Golgi cell–granule cell GABAergic synapse is immature in waggler mice. (A) Spontaneous GABAA responses with kynurenic acid (2 mM) in the bath solution. Each sweep represents a 1-s period, and the scale bar applies to all three traces. Response kinetics in adult waggler (P35) and young wild-type (P17) mice are slower than that of adult wild-type (P35). (B) Scaled spontaneous GABAA responses. Each trace is an average of 20 events. (C) Relative amplitude (in percent) and decay time constant of the two exponentials fitted to the decaying phase of the GABAA response. Each trace used in the fitting was an average of all events during a 1-min recording period. (D) In situ hybridization detected a 20% reduction of GABAA receptor α6 subunit mRNA level in adult waggler cerebellum. Shown here are typical examples.
Absence of AMPA Receptor Currents at waggler mf-Granule Cell Synapses.
A major feature of central synapse maturation is the transition from silent NMDA receptor-only glutamatergic synapses to functional NMDA-AMPA receptor synapses (35–38). The glutamatergic mf-granule cell synapses undergo a similar process during early postnatal development, in which NMDA receptor-mediated responses decrease and AMPA receptor-mediated responses increase (17). We compared AMPA receptor responses of wild-type and mutant mf-granule cell synapses. In adult wild types, AMPA receptor-mediated fast synaptic currents were prominent in every granule cell recorded (n = 8, see Fig. 4 A and C). In contrast, 12 of 19 cells recorded from developing (P17) wild-type granule cells showed small or no AMPA receptor EPSCs (Fig. 4C). The presence of voltage-activated Na+ currents was used to confirm that the recorded cells were neurons. Surprisingly, the fast EPSCs were missing in both developing (n = 11) and adult waggler mutant granule cells (n = 11; Fig. 4 A and C), whereas all of the 22 cells showed voltage-activated Na+ currents. To determine whether the absence of AMPA receptor responses was because of the absence of functional postsynaptic AMPA receptors, we recorded granule cell responses to exogenous AMPA. Pressure-ejected AMPA (500 μM, 1 s, 20 psi) produced inward currents in wild-type granule cells (n = 5), but not in mutant granule cells (n = 5, see Fig. 4B for examples), suggesting that no functional AMPA receptors were present in granule cell membranes. The deficit does not seem to be a general property of all cerebellar synapses because both pf- and climbing fiber-PC synapses exhibit AMPA receptor-mediated responses as mentioned before (also see Fig. 2). To determine whether the absence of AMPA responses was because of a lack of AMPA receptor expression, mRNA levels of GluR1–4 (both flip and flop) splice variants in adult mutant and wild-type mice were studied with in situ hybridization. No difference in mRNA levels was found between the wild-type and waggler cerebellar granule cell layers for any of the eight probes. Fig. 4D presents the autoradiographs for GluR4 (flop) mRNA, which is highly expressed in cerebellar granule cells. Our results indicate that the development of functional AMPA receptors at mf-granule cell synapses is impaired in wagglers.
Figure 4.
The AMPA receptor-mediated mf-granule cell synaptic currents are absent in waggler mice. (A) Current–voltage relation of AMPA receptor synaptic EPSCs in adult wild-type and waggler mice. The membrane potentials were from −80 mV to +80 mV with 20 mV steps. Recordings were done in the presence of d-2-amino-5-phosphonovaleric acid (APV; 100 μM) and picrotoxin (PTX; 40 μM). (B) Granule cell responses to exogenous AMPA. Wild-type granule cells, but not waggler granule cells, exhibited inward currents in response to AMPA puff (indicated by the bars). (C) AMPA receptor (AMPAR) EPSCs (Vm = −80 mV) at mf-granule cell synapses before and after CNQX (10 μM) application. Developing (P17) wild-type granule cells exhibited responses with a wide range of amplitudes indicating their asynchronized maturation, whereas adult wild-type (>P28) granule cells consistently showed large responses. waggler granule cells failed to develop AMPA receptor EPSCs. (D) GluR-4 mRNA levels.
NMDA Receptor Currents Are Present at waggler mf-Granule Cell Synapses.
The development of functional AMPA receptors appears to be NMDA receptor-dependent (35). To determine whether the absence of AMPA responses in mutant granule cells is because of impairment in NMDA receptor functions and whether mf transmitter release is impaired, we compared NMDA receptor-mediated synaptic responses in wild-type and waggler granule cells. In contrast to AMPA receptor-mediated responses, NMDA receptor-mediated responses were larger in developing waggler granule cells than in age-matched wild-type ones (Fig. 5C, waggler, n = 12; wild type, n = 16; P < 0.05) and exhibited normal Mg2+ blockade (Fig. 5 A and B). In adults, NMDA receptor-mediated responses decreased in both wild-type and waggler granule cells (Fig. 5C, waggler, n = 10; wild type, n = 10; P > 0.1). Thus, glutamate release at waggler cerebellar mf terminals is not impaired, and the failed development of functional AMPA receptors in waggler granule cells is not caused by impairment in NMDA receptor functions. The enhanced NMDA receptor currents in P17 waggler granule cells may reflect delayed developmental down-regulation of NMDA receptor currents.
Figure 5.
NMDA receptor-mediated mf-granule cell EPSCs are not impaired in waggler granule cells. (A) Current–voltage relation of NMDA receptor synaptic EPSCs in P17 wild-type (WT) and waggler granule cells. The membrane potentials were from −80 mV to + 60 mV with 20-mV steps. Note the wild-type responses were recorded in the presence of both CNQX and picrotoxin (PTX), whereas the waggler responses were recorded with PTX only. (B) Current–voltage curve of EPSCs shown in A. All the amplitudes were normalized to the response at +20 mV. The waggler mf-granule cell EPSCs are typical NMDA receptor-mediated responses. (C) NMDA receptor (NMDAR) EPSCs (Vm = +60 mV) at mf-granule cell synapses before and after d-2-amino-5-phosphonovaleric acid (APV; 100 μM) application. Both wild-type and waggler granule cells exhibited NMDA receptor-mediated responses, which decrease during development (from P17 to P28).
Prolonged BDNF Treatment Fails to Restore AMPA Receptor Response in waggler Granule Cells.
To address the possibility that impaired cerebellar granule cell synapse maturation in waggler mice is caused by cerebellar BDNF deficit, we attempted to rescue mutant granule cell synapses with BDNF incubation. P17 cerebellar slices were used because BDNF expression normally starts at about this age (2) and because some wild-type granule cells at this stage are still undergoing synapse maturation. We incubated cerebellar slices with BDNF (100 ng/ml in ACSF) for 12 hr as described (25). In wild-type slices, BDNF incubation did not result in significant changes in mf-granule cell AMPA receptor EPSCs (n = 9), as compared with those recorded from slices incubated with ACSF for 12 hr (n = 10). This is consistent with results of previous studies showing that neither prolonged BDNF incubation (25) nor virus-mediated BDNF gene transfer (39) enhanced hippocampal CA1 excitatory synaptic transmission in wild types. Further, waggler granule cells (n = 15) did not show any up-regulation of AMPA receptor EPSCs after 12 hr of incubation with BDNF, whereas the same cells all exhibited NMDA receptor-mediated EPSCs when the membrane potentials were held at +60 mV.
Discussion
In the present study, we show deficits in multiple types of granule cell synapses in adult waggler mutant mice. Although the abnormalities found in the waggler mutant mice seem to be diverse, all the deficits represent features of immature synapses. Because the lack of stargazin may increase Ca2+ influx at presynaptic terminals, it is also possible that the observed deficits in waggler cerebellar synapses are because of altered Ca2+ channels at presynaptic terminals. However, our results suggest that abnormal presynaptic Ca entry cannot fully account for the deficits. First, excitatory neurotransmitter release is decreased at pf-PC synapses, instead of increased as predicted by enhanced Ca2+ entry because of reduced Ca2+ channel inactivation (8). Second, the differential effects of the waggler mutation on the NMDA receptor- and AMPA receptor-mediated mf-granule cell synaptic responses suggest postsynaptic mechanisms. Third, developmental transition of the kinetics of GABAA receptor-mediated synaptic responses is generally believed to be mediated by postsynaptic mechanisms, as mentioned before.
The impaired granule cell synapse maturation may be caused by several factors including a granule cell-specific BDNF deficit and/or altered Ca2+ channel properties. Although BDNF has been shown to be involved in cerebellar development (40), our results indicate that normal granule cell BDNF expression is not essential for granule cell migration, differentiation, survival, or cerebellar foliation. The failure to rescue AMPA receptor synaptic responses with prolonged BDNF incubation in wagglers also suggests that BDNF deficit is not a direct cause of the deficiency in the mutant glutamatergic synapses. Alternatively, the lack of BDNF may be because of developmental arrest of the granule cells.
Activation of silent synapses may be a mechanism underlying long-term synaptic potentiation and maturation of glutamatergic synapses (35, 38, 41). AMPA receptor insertion and clustering in postsynaptic membranes have been considered as potential mechanisms for modulation of AMPA receptor responses (42–44). However, the intracellular signals that guide the proper targeting of AMPA receptors are yet to be discovered. Stargazin is highly expressed in cerebellar granule cells. Losing normal stargazin in wagglers would lead to reduced steady-state inactivation of Ca2+ currents in cerebellar granule cells and enhanced Ca2+ entry through VDCCs (8). L-type VDCCs are mainly located at the base of the major dendrites (45) and initiate Ca2+ signaling spatially distinct from that mediated by NMDA receptor (46–48). It has been shown that regulation of gene expression by intracellular Ca2+ depends on the spatial compartmentation of Ca2+ signaling (49, 50). In wagglers, the anticipated increase in nonsynaptic Ca2+ entry through deficient VDCC may disrupt the local Ca2+ signaling through synaptic NMDA receptors. The failure of synapse maturation in mutant granule cells suggests that such a signal may be one of the intracellular messengers that target AMPA receptors to postsynaptic membranes. This hypothesis is consistent with previous findings that NMDA receptor-independent long-term potentiation is generally presynaptic and does not involve modulation of postsynaptic AMPA receptors (51–54) and that activation of VDCCs often leads to long-term depression (55, 56). Similarly, depolarization and consequent activation of VDCC by blocking GABAA receptors down-regulate surface AMPA receptors, AMPA receptor EPSCs and AMPA receptor miniature EPSCs (57, 58).
Acknowledgments
We thank Drs. Michel Baudry and Lou Byerly for comments on the manuscript. The waggler breeding pairs were kindly provided by Dr. Verity A. Letts (The Jackson Laboratory). This work was supported by National Science Foundation Grant IBN-9215069, National Institute of Aging Grant AG-14751, National Institute of Mental Health Grant 5P20-MH52194, and a grant from Sankyo Company (to R.F.T.).
Abbreviations
- BDNF
brain-derived neurotrophic factor
- GABA
γ-aminobutyric acid
- NMDA
N-methyl-d-aspartate
- EPSC
excitatory postsynaptic current
- ACSF
artificial cerebrospinal fluid
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- VDCC
voltage-dependent calcium channel
- EPSP
excitatory postsynaptic potential
- PPF
paired-pulse facilitation
- pf-PC
parallel fiber–Purkinje cell
- mf
mossy fiber
- Pn
postnatal day n
References
- 1.Bao S, Chen L, Qiao X, Thompson R F. Learn Mem. 1998;5:355–364. [PMC free article] [PubMed] [Google Scholar]
- 2.Qiao X, Hefti F, Knusel B, Noebels J L. J Neurosci. 1996;16:640–648. doi: 10.1523/JNEUROSCI.16-02-00640.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rocamora N, Garcia-Ladona F J, Palacios J M, Mengod G. Mol Brain Res. 1993;17:1–8. doi: 10.1016/0169-328x(93)90065-w. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi M, Seagar M J, Jones J F, Beber B F X, Catterall W A. Proc Natl Acad Sci USA. 1987;84:5478–5482. doi: 10.1073/pnas.84.15.5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Catterall W A. Science. 1988;242:50–61. doi: 10.1126/science.2459775. [DOI] [PubMed] [Google Scholar]
- 6.Singer D, Biel M, Lotan I, Flockerzi V, Hofmann F, Dascal N. Science. 1991;253:1553–1557. doi: 10.1126/science.1716787. [DOI] [PubMed] [Google Scholar]
- 7.Wei X, Perez-Reyes E, Lacerda A E, Schuster G, Brown A M, Birnbaumer L. J Biol Chem. 1991;266:21943–21947. [PubMed] [Google Scholar]
- 8.Letts V A, Felix R, Biddlecome G H, Arikkath J, Mahaffey C L, Valenzuela A, Bartlett F S, 2nd, Mori Y, Campbell K P, Frankel W N. Nat Genet. 1998;19:340–347. doi: 10.1038/1228. [DOI] [PubMed] [Google Scholar]
- 9.Mellor J R, Merlo D, Jones A, Wisden W, Randall A D. J Neurosci. 1998;18:2822–2833. doi: 10.1523/JNEUROSCI.18-08-02822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gault L M, Siegal R E. J Neurosci. 1997;17:2391–2399. doi: 10.1523/JNEUROSCI.17-07-02391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuhar S, Feng L, Vidan S, Ross E R, Hatten M E, Heintz N. Development. 1993;112:97–104. doi: 10.1242/dev.117.1.97. [DOI] [PubMed] [Google Scholar]
- 12.Laurie D J, Wisden W, Seeburg P H. J Neurosci. 1992;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bessho Y, Nawa H, Nakanishi S. Neuron. 1994;12:87–95. doi: 10.1016/0896-6273(94)90154-6. [DOI] [PubMed] [Google Scholar]
- 14.Condorelli D F, Dell’Albani P, Aronica E, Genazzani A A, Casabona G, Corsaro M, Balazs R, Nicoletti F. J Neurochem. 1993;61:2133–2139. doi: 10.1111/j.1471-4159.1993.tb07451.x. [DOI] [PubMed] [Google Scholar]
- 15.Cox J A, Felder C C, Henneberry R C. Neuron. 1990;4:941–947. doi: 10.1016/0896-6273(90)90147-8. [DOI] [PubMed] [Google Scholar]
- 16.Vallano M L, Lambolez B, Audinat E, Rossier J. J Neurosci. 1996;16:631–639. doi: 10.1523/JNEUROSCI.16-02-00631.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D’Angelo E, Rossi P, Taglietti V. Neuroscience. 1993;53:121–130. doi: 10.1016/0306-4522(93)90290-v. [DOI] [PubMed] [Google Scholar]
- 18.Monyer H, Burnashev N, Laurie D J, Sakmann B, Seeburg P H. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi T, Feldmeyer D, Suzuki N, Onodera K, Cull-Candy S G, Sakimura K, Mishina M. J Neurosci. 1996;16:4376–4382. doi: 10.1523/JNEUROSCI.16-14-04376.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qiao X, Chen L, Gao H, Bao S, Hefti F, Thompson R F, Knusel B. J Neurosci. 1998;18:6990–6999. doi: 10.1523/JNEUROSCI.18-17-06990.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones K R, Farinas I, Backus C, Reichardt L F. Cell. 1994;76:989–999. doi: 10.1016/0092-8674(94)90377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sommer B, Keinanen K, Verdoorn T A, Wisden W, Burnashev N, Herb A, Kohler M, Takagi T, Sakmann B, Seeburg P H. Science. 1990;249:1580–1585. doi: 10.1126/science.1699275. [DOI] [PubMed] [Google Scholar]
- 23.Gallo V, Upson L M, Hayes W P, Vyklicky L, Jr, Winters C A, Buonanno A. J Neurosci. 1992;12:1010–1023. doi: 10.1523/JNEUROSCI.12-03-01010.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blanton E, Lo Turco J J, Kriegstein A R. J Neurosci Methods. 1989;30:203–210. doi: 10.1016/0165-0270(89)90131-3. [DOI] [PubMed] [Google Scholar]
- 25.Patterson S L, Abel T, Deuel T A, Martin K C, Rose J C, Kandel E R. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- 26.Hatten M E, Heintz N. Annu Rev Neurosci. 1995;18:385–408. doi: 10.1146/annurev.ne.18.030195.002125. [DOI] [PubMed] [Google Scholar]
- 27.Katz B, Miledi R. J Physiol. 1968;195:481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Atluri P P, Regehr W G. J Neurosci. 1996;16:5661–5671. doi: 10.1523/JNEUROSCI.16-18-05661.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manabe T, Wyllie D J, Perkel D J, Nicoll R A. J Neurophysiol. 1993;70:1451–1459. doi: 10.1152/jn.1993.70.4.1451. [DOI] [PubMed] [Google Scholar]
- 30.Fritschy J M, Paysan J, Enna A, Mohler H. J Neurosci. 1994;14:5302–5324. doi: 10.1523/JNEUROSCI.14-09-05302.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson C L, Stephenson F A. J Neurochem. 1994;62:2037–2044. doi: 10.1046/j.1471-4159.1994.62052037.x. [DOI] [PubMed] [Google Scholar]
- 32.Santi M R, Vicini S, Eldadah B, Neale J H. J Neurochem. 1994;63:2357–2360. doi: 10.1046/j.1471-4159.1994.63062357.x. [DOI] [PubMed] [Google Scholar]
- 33.Tia S, Wang J F, Kotchabhakdi N, Vicini S. J Neurosci. 1996;16:3630–3640. doi: 10.1523/JNEUROSCI.16-11-03630.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson C L, Tehrani M H J, Barnes E M, Jr, Stephenson F A. Mol Brain Res. 1998;60:282–290. doi: 10.1016/s0169-328x(98)00205-8. [DOI] [PubMed] [Google Scholar]
- 35.Durand G M, Kovalchuk Y, Konnerth A. Nature (London) 1996;381:71–74. doi: 10.1038/381071a0. [DOI] [PubMed] [Google Scholar]
- 36.Hsia A Y, Malenka R C, Nicoll R A. J Neurophysiol. 1998;79:2013–2024. doi: 10.1152/jn.1998.79.4.2013. [DOI] [PubMed] [Google Scholar]
- 37.Wu G-Y, Malinow R, Cline H T. Science. 1996;274:972–976. doi: 10.1126/science.274.5289.972. [DOI] [PubMed] [Google Scholar]
- 38.Issac J T R, Crair M C, Nicoll R A, Malenka R C. Neuron. 1997;18:269–280. doi: 10.1016/s0896-6273(00)80267-6. [DOI] [PubMed] [Google Scholar]
- 39.Korte M, Griesbeck O, Gravel C, Carroll P, Staiger V, Thoenen H, Bonhoeffer T. Proc Natl Acad Sci USA. 1996;93:12547–12552. doi: 10.1073/pnas.93.22.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwartz P M, Borghesani P R, Levy R L, Pomeroy S L, Segal R A. Neuron. 1997;19:269–281. doi: 10.1016/s0896-6273(00)80938-1. [DOI] [PubMed] [Google Scholar]
- 41.Liao D, Hessler N A, Malinow R C. Nature (London) 1995;375:400–404. doi: 10.1038/375400a0. [DOI] [PubMed] [Google Scholar]
- 42.Lledo P M, Zhang X, Sudhof T C, Malenka R C, Nicoll R A. Science. 1998;279:399–403. doi: 10.1126/science.279.5349.399. [DOI] [PubMed] [Google Scholar]
- 43.Lynch G, Baudry M. Hippocampus. 1991;1:9–14. doi: 10.1002/hipo.450010103. [DOI] [PubMed] [Google Scholar]
- 44.Xie X, Liaw J-S, Baudry M, Berger T D. Proc Natl Acad Sci USA. 1997;94:6983–6988. doi: 10.1073/pnas.94.13.6983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Westenbroek R E, Ahlijanian M K, Catterall W A. Nature (London) 1990;347:281–284. doi: 10.1038/347281a0. [DOI] [PubMed] [Google Scholar]
- 46.Guthrie P B, Segal M, Kater S B. Nature (London) 1991;354:76–80. doi: 10.1038/354076a0. [DOI] [PubMed] [Google Scholar]
- 47.Müller W, Connor J A. Nature (London) 1991;354:73–76. doi: 10.1038/354073a0. [DOI] [PubMed] [Google Scholar]
- 48.Regehr W G, Tank D W. Nature (London) 1990;345:807–810. doi: 10.1038/345807a0. [DOI] [PubMed] [Google Scholar]
- 49.Finkbeiner S, Greenberg M E. BioEssays. 1997;19:657–660. doi: 10.1002/bies.950190803. [DOI] [PubMed] [Google Scholar]
- 50.Ginty D D. Neuron. 1997;18:183–186. doi: 10.1016/s0896-6273(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 51.Linden D J. Neuron. 1997;18:983–994. doi: 10.1016/s0896-6273(00)80337-2. [DOI] [PubMed] [Google Scholar]
- 52.Nicoll R A, Malenka R C. Nature (London) 1995;377:115–118. doi: 10.1038/377115a0. [DOI] [PubMed] [Google Scholar]
- 53.Salin P A, Malenka R C, Nicoll R A. Neuron. 1996;16:797–803. doi: 10.1016/s0896-6273(00)80099-9. [DOI] [PubMed] [Google Scholar]
- 54.Weisskopf M G, Castillo P E, Zalutsky R A, Nicoll R A. Science. 1994;265:1878–1882. doi: 10.1126/science.7916482. [DOI] [PubMed] [Google Scholar]
- 55.Bolshakov V Y, Siegelbaum S A. Science. 1994;264:1148–1152. doi: 10.1126/science.7909958. [DOI] [PubMed] [Google Scholar]
- 56.Cummings J A, Mulkey R M, Nicoll R A, Malenka R C. Neuron. 1996;16:825–833. doi: 10.1016/s0896-6273(00)80102-6. [DOI] [PubMed] [Google Scholar]
- 57.Lissin D V, Gomperts S N, Carroll R C, Christine C W, Kalman D, Kitamura M, Hardy S, Nicoll R A, Malenka R C, von Zastrow M. Proc Natl Acad Sci USA. 1998;95:7097–7102. doi: 10.1073/pnas.95.12.7097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Turrigiano G G, Leslie K R, Desai N S, Rutherford L C, Nelson S B. Nature (London) 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]