Abstract
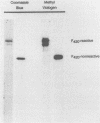
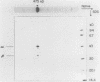
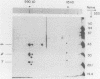
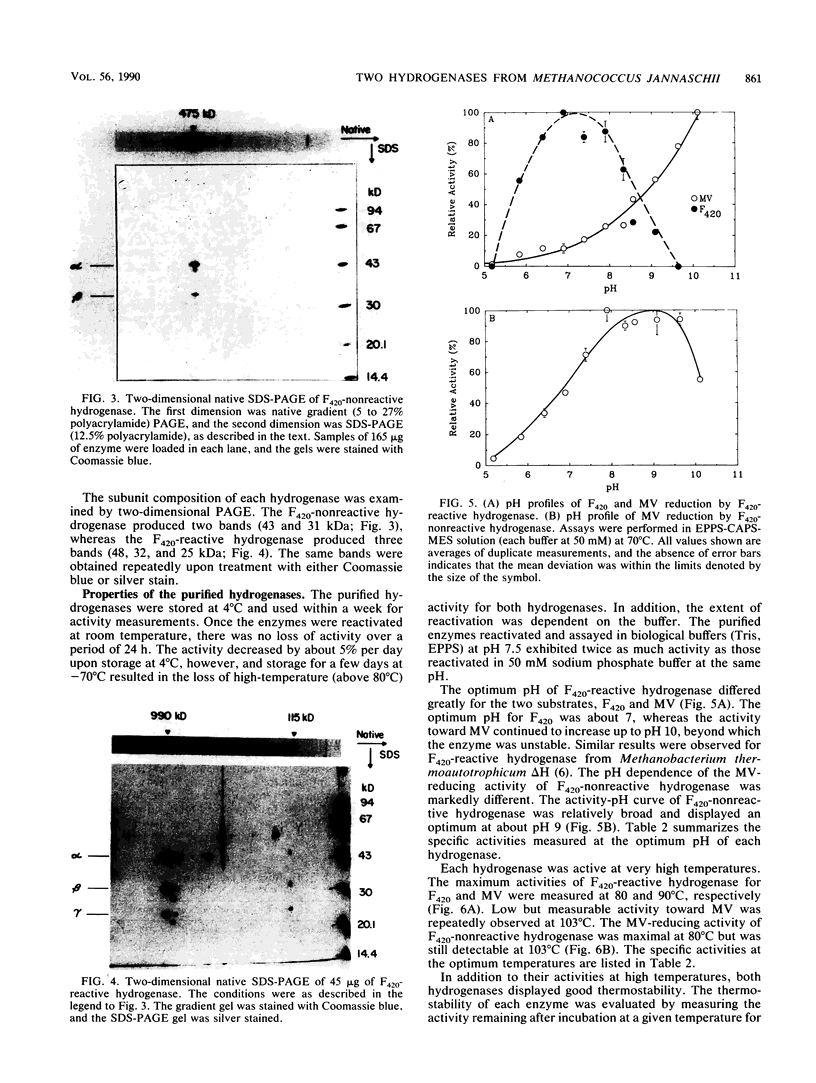
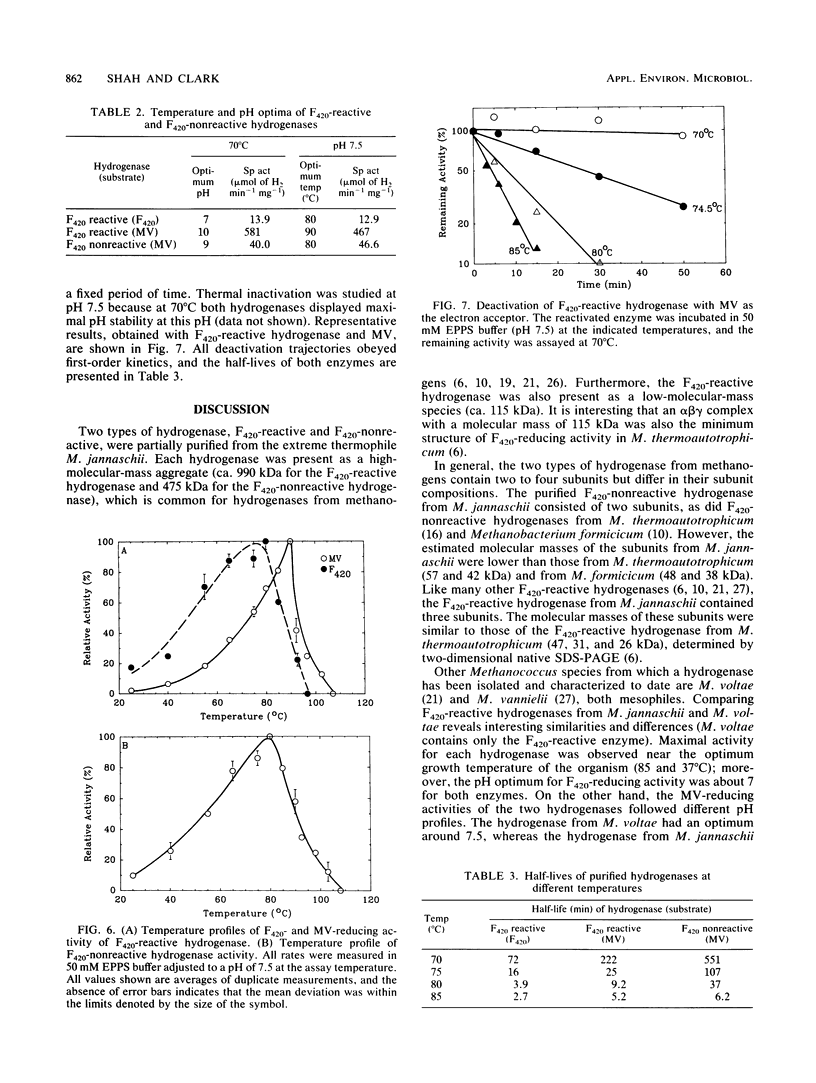
F420-nonreactive and F420-reactive hydrogenases have been partially purified from Methanococcus jannaschii, an extremely thermophilic methanogen isolated from a submarine hydrothermal vent. The molecular weights of both hydrogenases were determined by native gradient electrophoresis in 5 to 27% polyacrylamide gels. The F420-nonreactive hydrogenase produced one major band (475 kilodaltons), whereas the F420-reactive hydrogenase produced two major bands (990 and 115 kilodaltons). The F420-nonreactive hydrogenase consisted of two subunits (43 and 31 kilodaltons), and the F420-reactive hydrogenase contained three subunits (48, 32, and 25 kilodaltons). Each hydrogenase was active at very high temperatures. Methyl viologen-reducing activity of the F420-nonreactive hydrogenase was maximal at 80°C but was still detectable at 103°C. The maximum activities of F420-reactive hydrogenase for F420 and methyl viologen were measured at 80 and 90°C, respectively. Low but measureable activity toward methyl viologen was repeatedly observed at 103°C. Moreover, the half-life of the F420-nonreactive hydrogenase at 70°C was over 9 h, and that of the F420-reactive enzyme was over 3 h.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. W., Mortenson L. E., Chen J. S. Hydrogenase. Biochim Biophys Acta. 1980 Dec;594(2-3):105–176. doi: 10.1016/0304-4173(80)90007-5. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Drake H. L. Demonstration of hydrogenase in extracts of the homoacetate-fermenting bacterium Clostridium thermoaceticum. J Bacteriol. 1982 May;150(2):702–709. doi: 10.1128/jb.150.2.702-709.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. A., Livingston D. J., Orme-Johnson W. H., Walsh C. T. 8-Hydroxy-5-deazaflavin-reducing hydrogenase from Methanobacterium thermoautotrophicum: 1. Purification and characterization. Biochemistry. 1987 Jul 14;26(14):4219–4227. doi: 10.1021/bi00388a007. [DOI] [PubMed] [Google Scholar]
- Gogotov I. N., Zorin N. A., Serebriakova L. T., Kondratieva E. N. The properties of hydrogenase from Thiocapsa roseopersicina. Biochim Biophys Acta. 1978 Apr 12;523(2):335–343. doi: 10.1016/0005-2744(78)90036-0. [DOI] [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Jacobson F. S., Daniels L., Fox J. A., Walsh C. T., Orme-Johnson W. H. Purification and properties of an 8-hydroxy-5-deazaflavin-reducing hydrogenase from Methanobacterium thermoautotrophicum. J Biol Chem. 1982 Apr 10;257(7):3385–3388. [PubMed] [Google Scholar]
- Klibanov A. M. Enzyme stabilization by immobilization. Anal Biochem. 1979 Feb;93(1):1–25. [PubMed] [Google Scholar]
- Kojima N., Fox J. A., Hausinger R. P., Daniels L., Orme-Johnson W. H., Walsh C. Paramagnetic centers in the nickel-containing, deazaflavin-reducing hydrogenase from Methanobacterium thermoautotrophicum. Proc Natl Acad Sci U S A. 1983 Jan;80(2):378–382. doi: 10.1073/pnas.80.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappi D. A., Stolzenbach F. E., Kaplan N. O., Kamen M. D. Immobilization of hydrogenase on glass beads. Biochem Biophys Res Commun. 1976 Apr 19;69(4):878–884. doi: 10.1016/0006-291x(76)90455-1. [DOI] [PubMed] [Google Scholar]
- McKellar R. C., Sprott G. D. Solubilization and properties of a particulate hydrogenase from Methanobacterium strain G2R. J Bacteriol. 1979 Jul;139(1):231–238. doi: 10.1128/jb.139.1.231-238.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. F., Shah N. N., Nelson C. M., Ludlow J. M., Clark D. S. Pressure and Temperature Effects on Growth and Methane Production of the Extreme Thermophile Methanococcus jannaschii. Appl Environ Microbiol. 1988 Dec;54(12):3039–3042. doi: 10.1128/aem.54.12.3039-3042.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muth E., Mörschel E., Klein A. Purification and characterization of an 8-hydroxy-5-deazaflavin-reducing hydrogenase from the archaebacterium Methanococcus voltae. Eur J Biochem. 1987 Dec 15;169(3):571–577. doi: 10.1111/j.1432-1033.1987.tb13647.x. [DOI] [PubMed] [Google Scholar]
- Nelson M. J., Brown D. P., Ferry J. G. FAD requirement for the reduction of coenzyme F420 by hydrogenase from Methanobacterium formicicum. Biochem Biophys Res Commun. 1984 May 16;120(3):775–781. doi: 10.1016/s0006-291x(84)80174-6. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pihl T. D., Schicho R. N., Kelly R. M., Maier R. J. Characterization of hydrogen-uptake activity in the hyperthermophile Pyrodictium brockii. Proc Natl Acad Sci U S A. 1989 Jan;86(1):138–141. doi: 10.1073/pnas.86.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S. A selenium-containing hydrogenase from Methanococcus vannielii. Identification of the selenium moiety as a selenocysteine residue. J Biol Chem. 1982 Jul 25;257(14):7926–7929. [PubMed] [Google Scholar]