Abstract
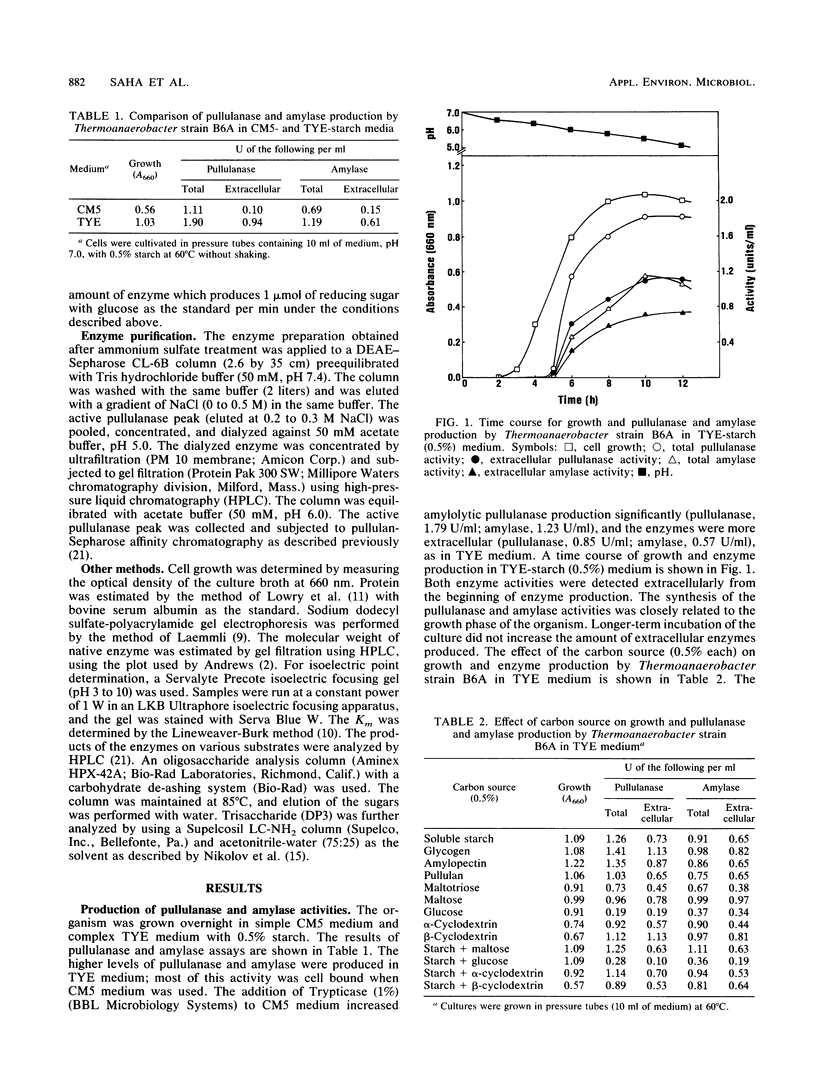
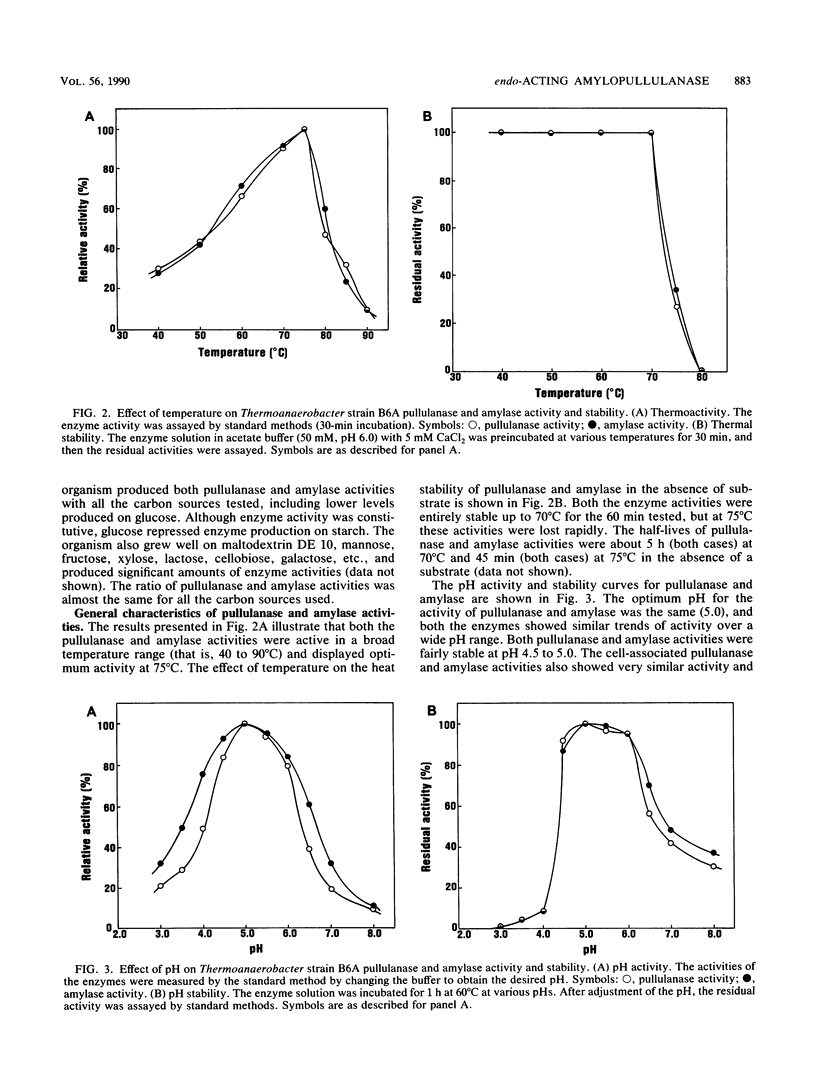
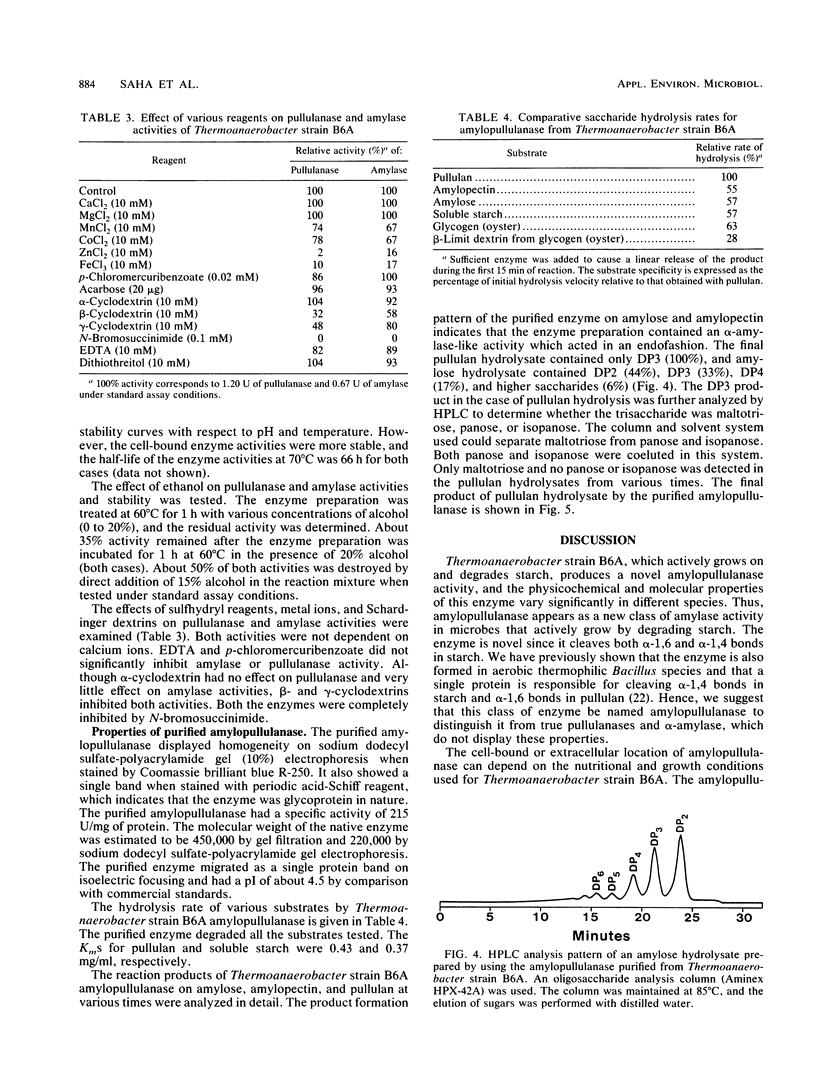
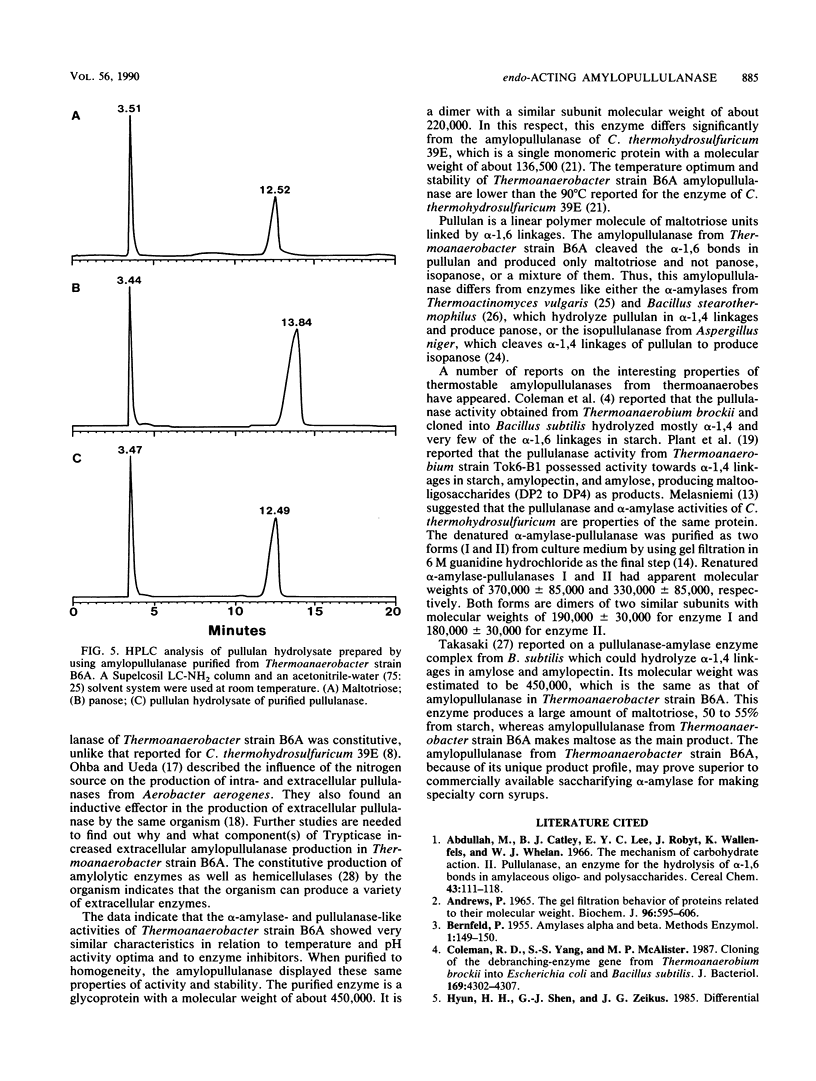
A thermoanaerobe (Thermoanaerobacter sp.) grown in TYE-starch (0.5%) medium at 60°C produced both extra- and intracellular pullulanase (1.90 U/ml) and amylase (1.19 U/ml) activities. Both activities were produced at high levels on a variety of carbon sources. The temperature and pH optima for both pullulanase and amylase activities were 75°C and pH 5.0, respectively. Both the enzyme activities were stable up to 70°C (without substrate) and at pH 4.5 to 5.0. The half-lives of both enzyme activities were 5 h at 70°C and 45 min at 75°C. The enzyme activities did not show any metal ion activity, and both activities were inhibited by β- and γ-cyclodextrins but not by α-cyclodextrin. A single amylolytic pullulanase responsible for both activities was purified to homogeneity by DEAE-Sepharose CL-6B column chromatography, gel filtration using high-pressure liquid chromatography, and pullulan-Sepharose affinity chromatography. It was a 450,000-molecular-weight glycoprotein composed of two equivalent subunits. The pullulanase cleaved pullulan in α1,6 linkages and produced multiple saccharides from cleavage of α-1,4 linkages in starch. The Kms for pullulan and soluble starch were 0.43 and 0.37 mg/ml, respectively.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman R. D., Yang S. S., McAlister M. P. Cloning of the debranching-enzyme gene from Thermoanaerobium brockii into Escherichia coli and Bacillus subtilis. J Bacteriol. 1987 Sep;169(9):4302–4307. doi: 10.1128/jb.169.9.4302-4307.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun H. H., Zeikus J. G. General Biochemical Characterization of Thermostable Extracellular beta-Amylase from Clostridium thermosulfurogenes. Appl Environ Microbiol. 1985 May;49(5):1162–1167. doi: 10.1128/aem.49.5.1162-1167.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun H. H., Zeikus J. G. General Biochemical Characterization of Thermostable Pullulanase and Glucoamylase from Clostridium thermohydrosulfuricum. Appl Environ Microbiol. 1985 May;49(5):1168–1173. doi: 10.1128/aem.49.5.1168-1173.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun H. H., Zeikus J. G. Regulation and genetic enhancement of glucoamylase and pullulanase production in Clostridium thermohydrosulfuricum. J Bacteriol. 1985 Dec;164(3):1146–1152. doi: 10.1128/jb.164.3.1146-1152.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Madi E., Antranikian G., Ohmiya K., Gottschalk G. Thermostable amylolytic enzymes from a new clostridium isolate. Appl Environ Microbiol. 1987 Jul;53(7):1661–1667. doi: 10.1128/aem.53.7.1661-1667.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melasniemi H. Characterization of alpha-amylase and pullulanase activities of Clostridium thermohydrosulfuricum. Evidence for a novel thermostable amylase. Biochem J. 1987 Aug 15;246(1):193–197. doi: 10.1042/bj2460193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melasniemi H. Purification and some properties of the extracellular alpha-amylase-pullulanase produced by Clostridium thermohydrosulfuricum. Biochem J. 1988 Mar 15;250(3):813–818. doi: 10.1042/bj2500813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha B. C., Mathupala S. P., Zeikus J. G. Purification and characterization of a highly thermostable novel pullulanase from Clostridium thermohydrosulfuricum. Biochem J. 1988 Jun 1;252(2):343–348. doi: 10.1042/bj2520343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakano Y., Higuchi M., Kobayashi T. Pullulan 4-glucanohydrolase from Aspergillus niger. Arch Biochem Biophys. 1972 Nov;153(1):180–187. doi: 10.1016/0003-9861(72)90434-1. [DOI] [PubMed] [Google Scholar]
- Weimer P. J., Wagner L. W., Knowlton S., Ng T. K. Thermophilic anaerobic bacteria which ferment hemicellulose: characterization of organisms and identification of plasmids. Arch Microbiol. 1984 May;138(1):31–36. doi: 10.1007/BF00425403. [DOI] [PubMed] [Google Scholar]
- Whelan W. J. Enzymic explorations of the structures of starch and glycogen. Biochem J. 1971 May;122(5):609–622. doi: 10.1042/bj1220609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G., Ben-Bassat A., Hegge P. W. Microbiology of methanogenesis in thermal, volcanic environments. J Bacteriol. 1980 Jul;143(1):432–440. doi: 10.1128/jb.143.1.432-440.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]


