Abstract
The mini Mu element Mu dII1681, which contains the lac operon genes and a kanamycin resistance gene, was inserted in the chromosome of plant growth-beneficial Pseudomonas aeruginosa 7NSK2 to construct a marked strain (MPB1). In MPB1, beta-galactosidase is permanently expressed under the culture conditions used. The MPB1 strain could be recovered with an efficiency of about 100% from a sandy loam soil on 5-bromo-4-chloro-3-indolyl-beta-D-galactopyranoside medium containing sebacic acid and kanamycin. The limit of detection is about 10 CFU/g of soil. A detailed comparison was made between the wild-type strain 7NSK2 and the Mu dII1681-containing MPB1 strain. The results showed that no genes essential for growth, siderophore production, survival in sterile and nonsterile conditions, plant growth stimulation, or root colonization had been damaged in the MPB1 strain, which means that MPB1 can reliably be used for ecological studies in soil. MPB1 survived well at 4 or 28 degrees C but died off relatively rapidly in air-dried soil or at subzero temperatures. In these conditions, however, the MPB1 strain did not completely disappear from the soil but survived at a very low level of about 100 CFU/g of soil for more than 3 months. This observation stresses the need for very sensitive counting methods for ecological studies and for the evaluation of released microorganisms. Maize was inoculated with MPB1 via seed inoculation or soil inoculation. Upon seed inoculation, only the upper root parts were effectively colonized, while soil inoculation resulted in a complete colonization of the root system.
Full text
PDF

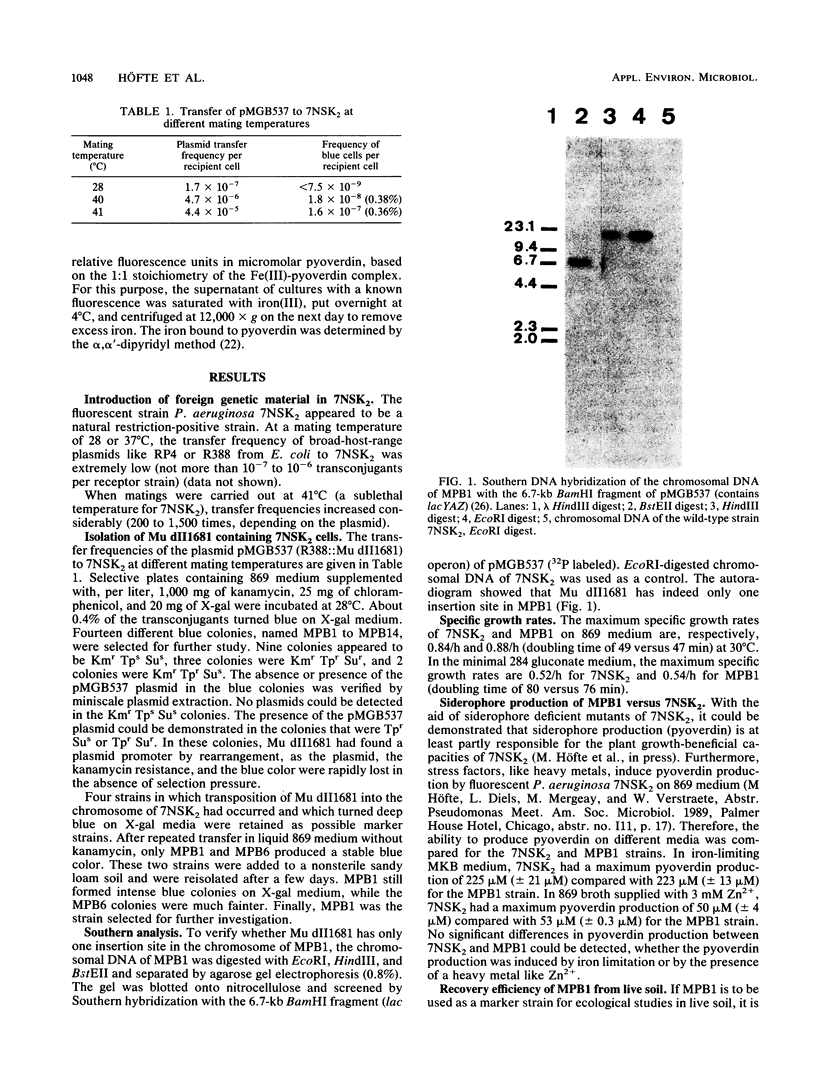
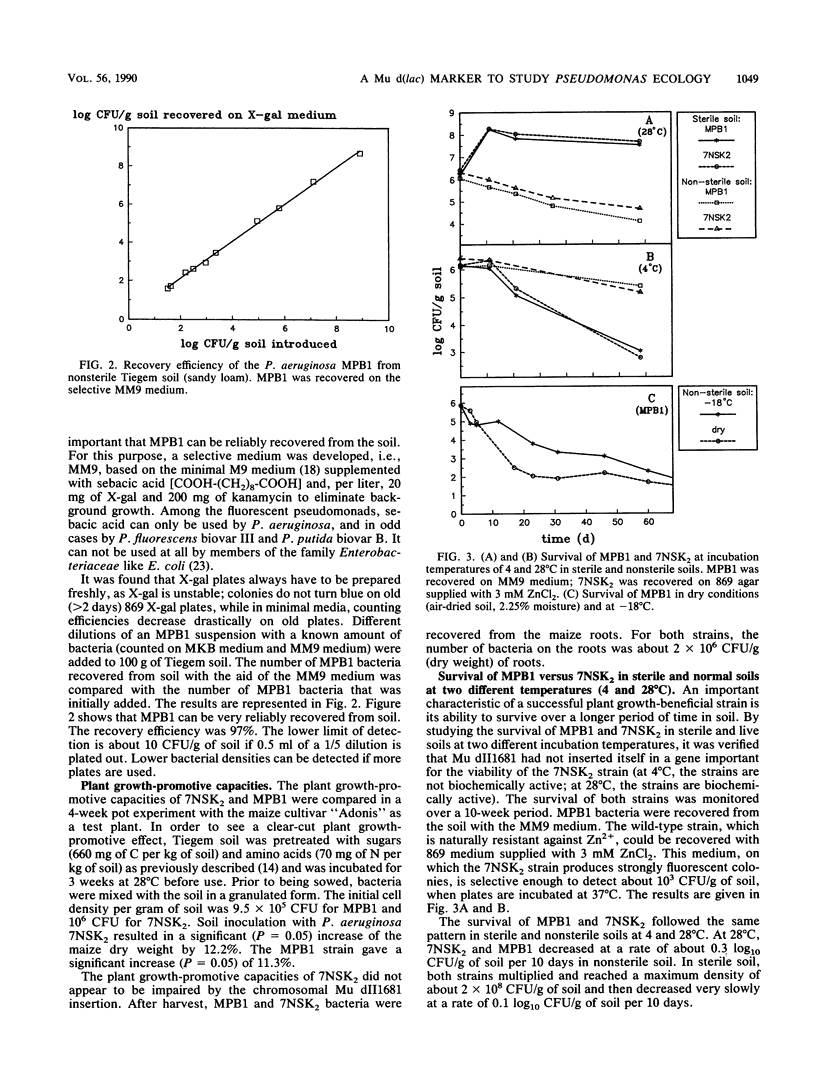
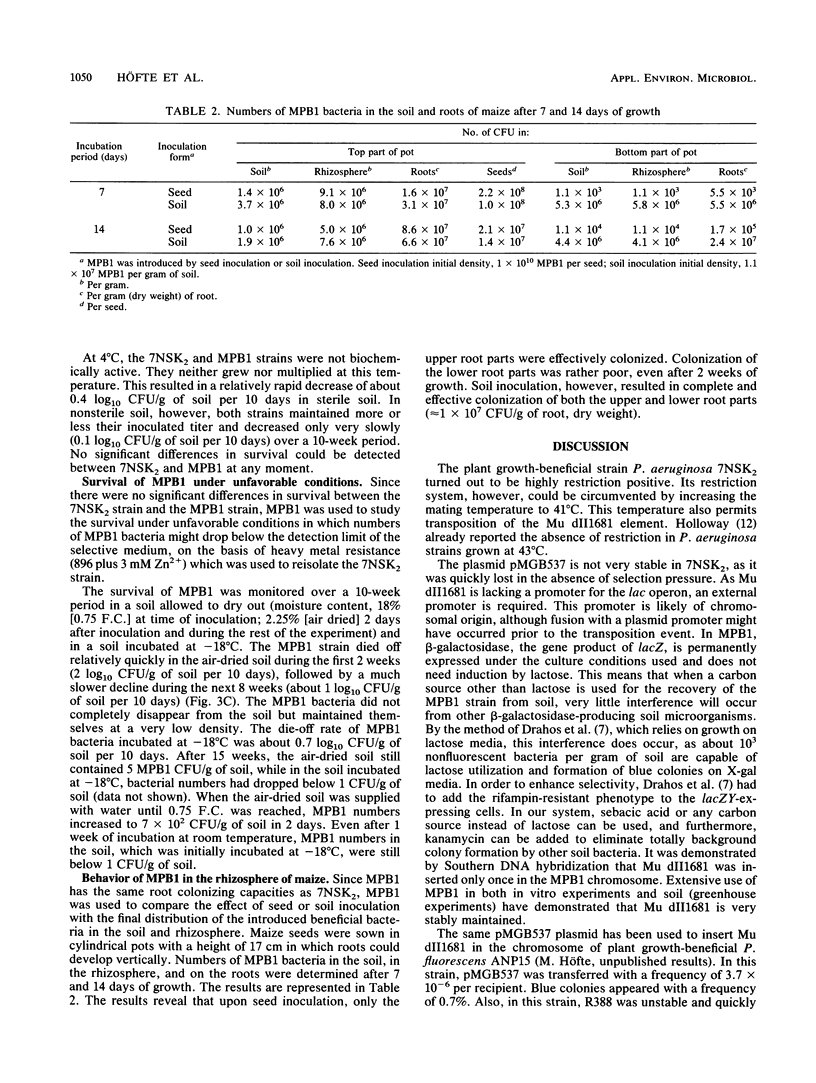


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Castilho B. A., Olfson P., Casadaban M. J. Plasmid insertion mutagenesis and lac gene fusion with mini-mu bacteriophage transposons. J Bacteriol. 1984 May;158(2):488–495. doi: 10.1128/jb.158.2.488-495.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Effect of growth conditions on the formation of the relaxation complex of supercoiled ColE1 deoxyribonucleic acid and protein in Escherichia coli. J Bacteriol. 1972 Jun;110(3):1135–1146. doi: 10.1128/jb.110.3.1135-1146.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compeau G., Al-Achi B. J., Platsouka E., Levy S. B. Survival of rifampin-resistant mutants of Pseudomonas fluorescens and Pseudomonas putida in soil systems. Appl Environ Microbiol. 1988 Oct;54(10):2432–2438. doi: 10.1128/aem.54.10.2432-2438.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson J. K., Bezdicek D. F., Brockman F. J., Li S. W. Enumeration of Tn5 mutant bacteria in soil by using a most- probable-number-DNA hybridization procedure and antibiotic resistance. Appl Environ Microbiol. 1988 Feb;54(2):446–453. doi: 10.1128/aem.54.2.446-453.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejeune P., Mergeay M., Van Gijsegem F., Faelen M., Gerits J., Toussaint A. Chromosome transfer and R-prime plasmid formation mediated by plasmid pULB113 (RP4::mini-Mu) in Alcaligenes eutrophus CH34 and Pseudomonas fluorescens 6.2. J Bacteriol. 1983 Sep;155(3):1015–1026. doi: 10.1128/jb.155.3.1015-1026.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergeay M., Lejeune P., Sadouk A., Gerits J., Fabry L. Shuttle transfer (or retrotransfer) of chromosomal markers mediated by plasmid pULB113. Mol Gen Genet. 1987 Aug;209(1):61–70. doi: 10.1007/BF00329837. [DOI] [PubMed] [Google Scholar]
- Mergeay M., Nies D., Schlegel H. G., Gerits J., Charles P., Van Gijsegem F. Alcaligenes eutrophus CH34 is a facultative chemolithotroph with plasmid-bound resistance to heavy metals. J Bacteriol. 1985 Apr;162(1):328–334. doi: 10.1128/jb.162.1.328-334.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osaki S., Johnson D. A., Frieden E. The mobilization of iron from the perfused mammalian liver by a serum copper enzyme, ferroxidase I. J Biol Chem. 1971 May 10;246(9):3018–3023. [PubMed] [Google Scholar]
- Simon V., Schumann W. In vivo formation of gene fusions in Pseudomonas putida and construction of versatile broad-host-range vectors for direct subcloning of Mu d1 and Mu d2 fusions. Appl Environ Microbiol. 1987 Jul;53(7):1649–1654. doi: 10.1128/aem.53.7.1649-1654.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffan R. J., Atlas R. M. DNA amplification to enhance detection of genetically engineered bacteria in environmental samples. Appl Environ Microbiol. 1988 Sep;54(9):2185–2191. doi: 10.1128/aem.54.9.2185-2191.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]