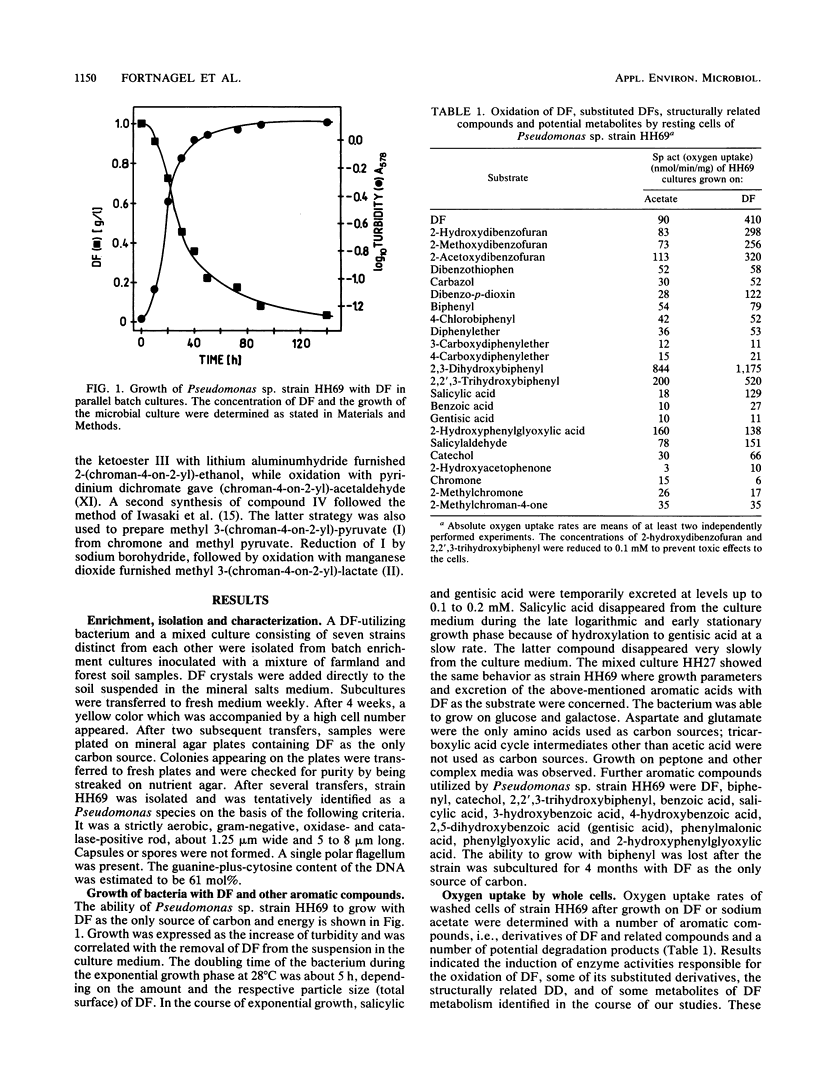
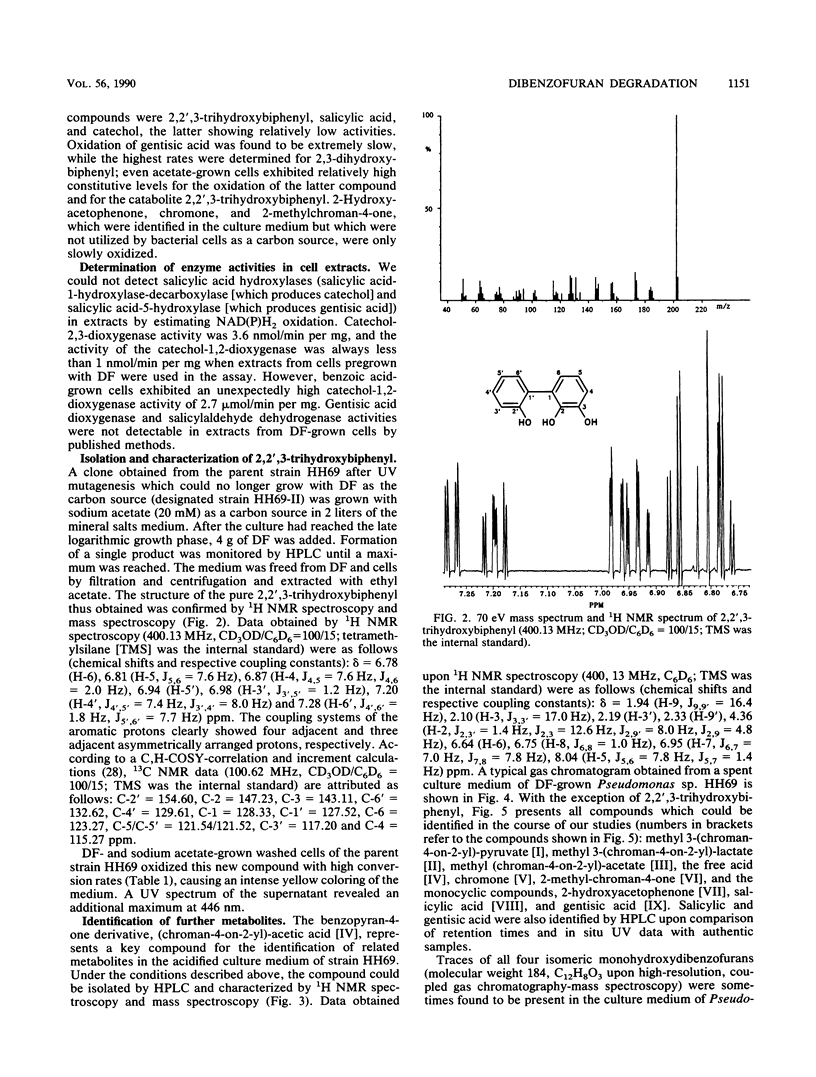
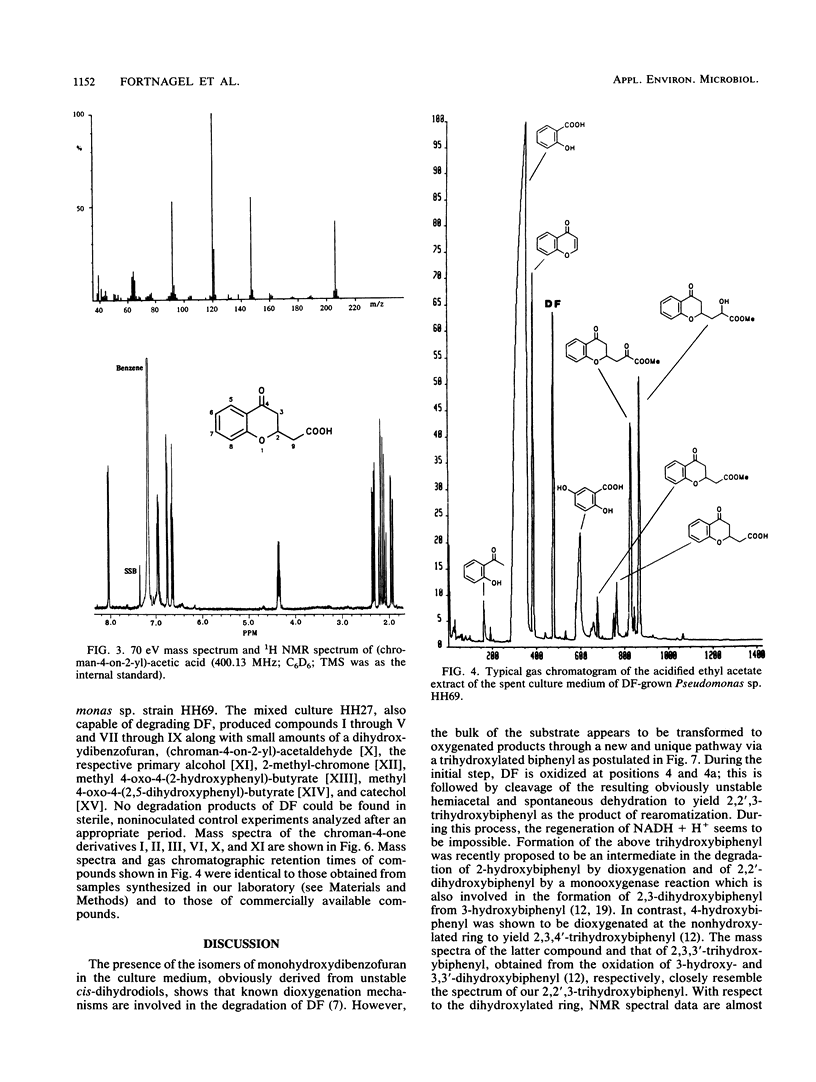
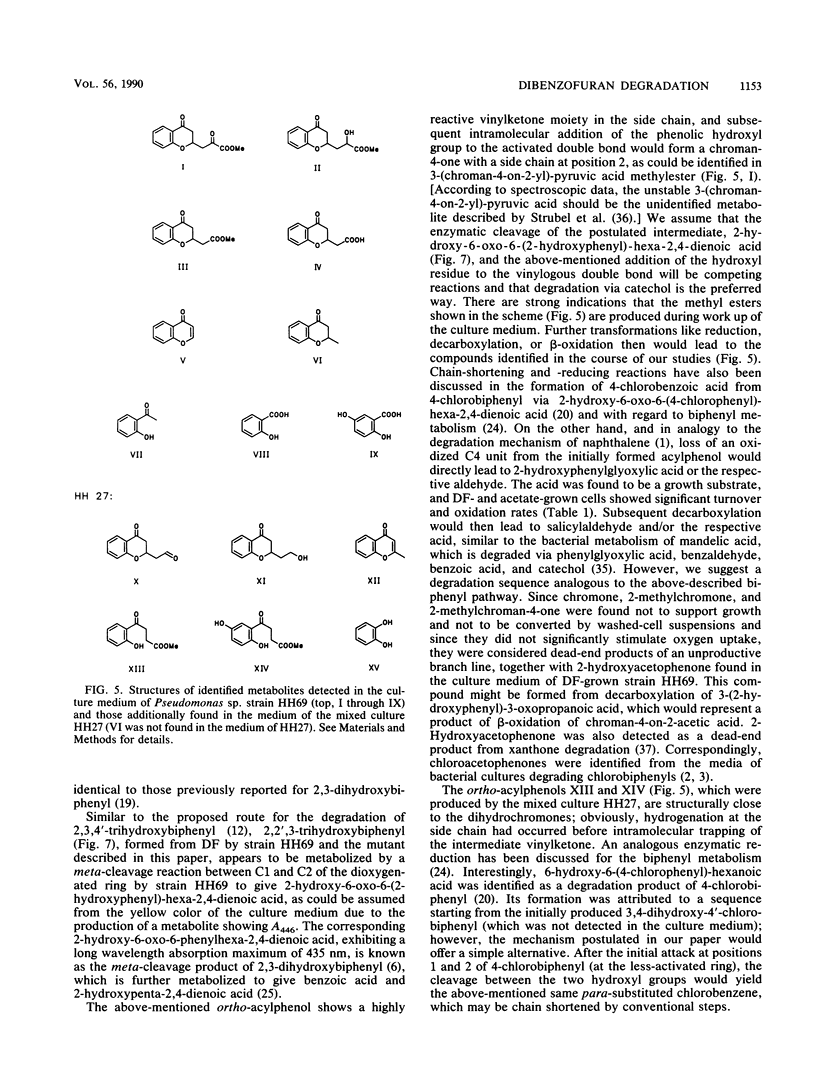
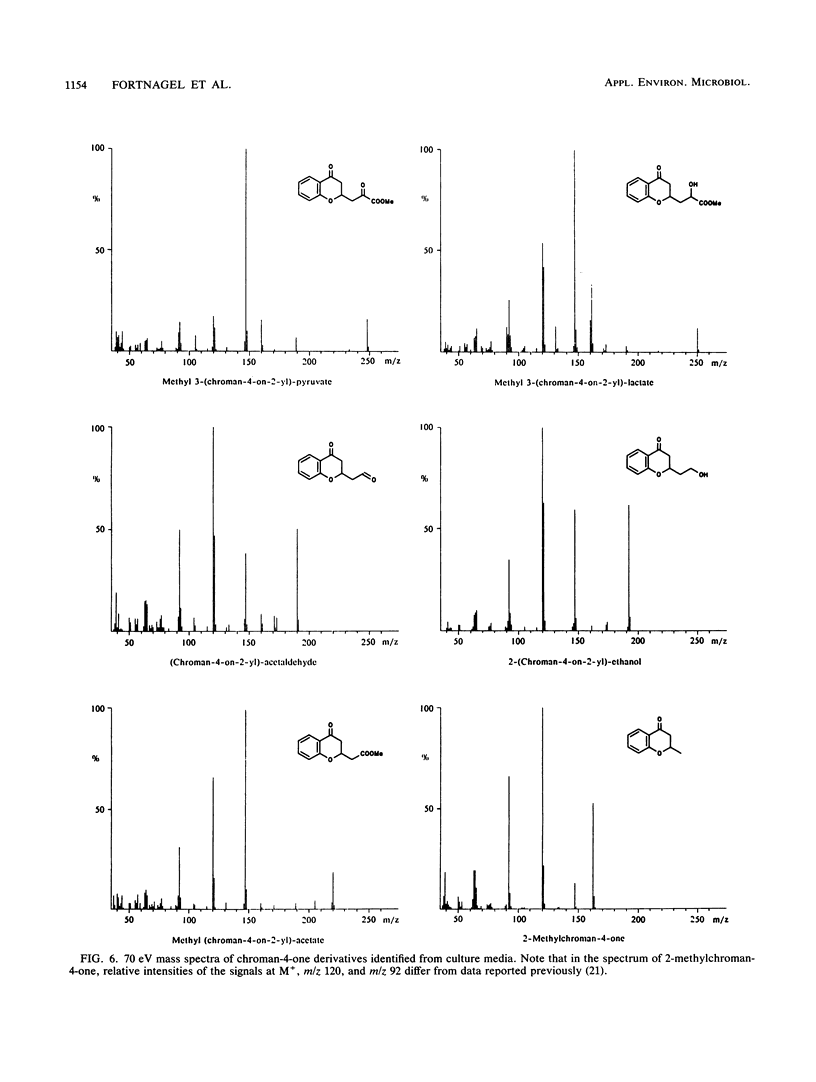
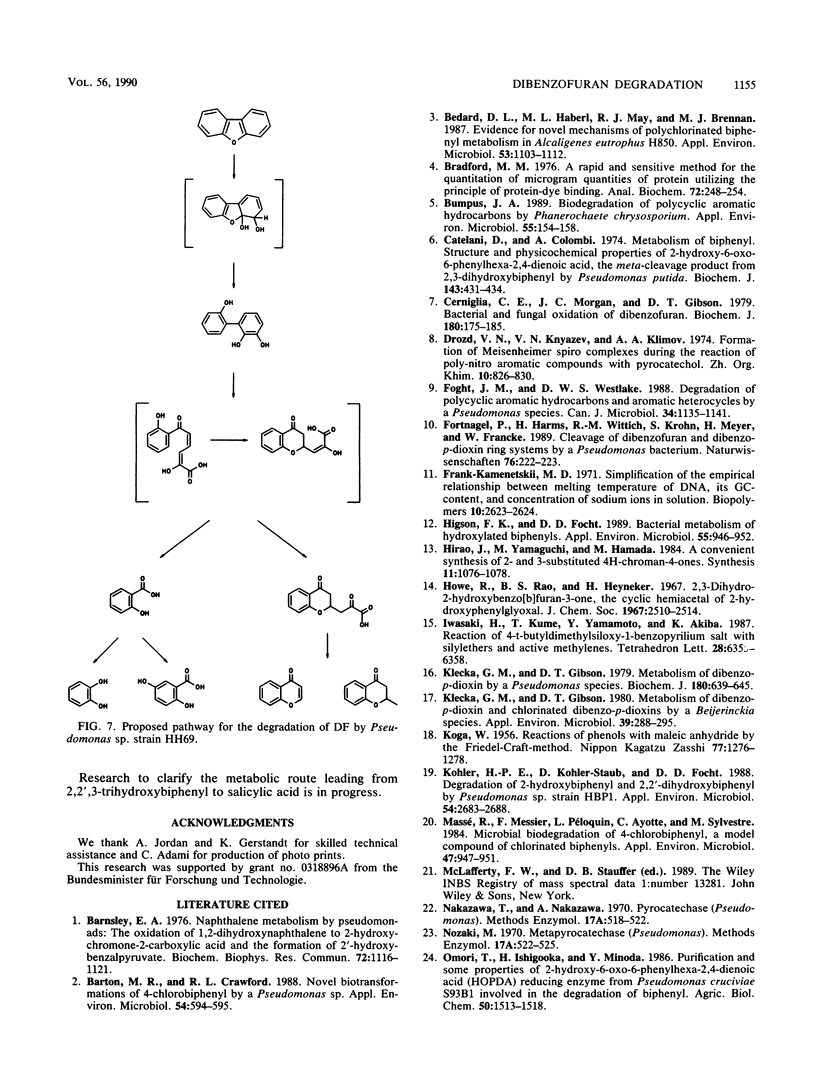
Abstract
A Pseudomonas sp. strain, HH69, and a mixed culture, designated HH27, were isolated by selective enrichment from soil samples. The pure strain and the mixed culture grew aerobically on dibenzofuran as the sole source of carbon and energy. Degradation proceeded via salicylic acid which was branched into the gentisic acid and the catechol pathway. Both salicylic acid and gentisic acid accumulated in the culture medium of strain HH69. The acids were slowly metabolized after growth ceased. The enzymes responsible for their metabolism showed relatively low activities. Besides the above-mentioned acids, 2-hydroxyacetophenone, benzopyran-4-one (chromone), several 2-substituted chroman-4-ones, and traces of the four isomeric monohydroxydiben-zofurans were identified in the culture medium. 2,2′,3-Trihydroxybiphenyl was isolated from the medium of a dibenzofuran-converting mutant derived from parent strain HH69, which can no longer grow on dibenzofuran. This gives evidence for a novel type of dioxygenases responsible for the attack on the biarylether structure of the dibenzofuran molecule. A meta-fission mechanism for cleavage of the dihydroxylated aromatic nucleus of 2,2′,3-trihydroxybiphenyl is suggested as the next enzymatic step in the degradative pathway.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnsley E. A. Naphthalene metabolism by pseudomonads: the oxidation of 1,2-dihydroxynaphthalene to 2-hydroxychromene-2-carboxylic acid and the formation of 2'-hydroxybenzalpyruvate. Biochem Biophys Res Commun. 1976 Oct 4;72(3):1116–1121. doi: 10.1016/s0006-291x(76)80247-1. [DOI] [PubMed] [Google Scholar]
- Barton M. R., Crawford R. L. Novel biotransformations of 4-chlorobiphenyl by a Pseudomonas sp. Appl Environ Microbiol. 1988 Feb;54(2):594–595. doi: 10.1128/aem.54.2.594-595.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard D. L., Haberl M. L., May R. J., Brennan M. J. Evidence for novel mechanisms of polychlorinated biphenyl metabolism in Alcaligenes eutrophus H850. Appl Environ Microbiol. 1987 May;53(5):1103–1112. doi: 10.1128/aem.53.5.1103-1112.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bumpus J. A. Biodegradation of polycyclic hydrocarbons by Phanerochaete chrysosporium. Appl Environ Microbiol. 1989 Jan;55(1):154–158. doi: 10.1128/aem.55.1.154-158.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catelani D., Colombi A. Metabolism of biphenyl. Structure and physicochemical properties of 2-hydroxy-6-oxo-6-phenylhexa-2,4-dienoic acid, the meta-cleavage product from 2,3-dihydroxybiphenyl by Pseudomonas putida. Biochem J. 1974 Nov;143(2):431–434. doi: 10.1042/bj1430431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerniglia C. E., Morgan J. C., Gibson D. T. Bacterial and fungal oxidation of dibenzofuran. Biochem J. 1979 Apr 15;180(1):175–185. doi: 10.1042/bj1800175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foght J. M., Westlake D. W. Degradation of polycyclic aromatic hydrocarbons and aromatic heterocycles by a Pseudomonas species. Can J Microbiol. 1988 Oct;34(10):1135–1141. doi: 10.1139/m88-200. [DOI] [PubMed] [Google Scholar]
- Fortnagel P., Harms H., Wittich R. M., Francke W., Krohn S., Meyer H. Cleavage of dibenzofuran and dibenzodioxin ring systems by a Pseudomonas bacterium. Naturwissenschaften. 1989 May;76(5):222–223. doi: 10.1007/BF00627694. [DOI] [PubMed] [Google Scholar]
- Frank-Kamenetskii F. Simplification of the empirical relationship between melting temperature of DNA, its GC content and concentration of sodium ions in solution. Biopolymers. 1971;10(12):2623–2624. doi: 10.1002/bip.360101223. [DOI] [PubMed] [Google Scholar]
- Higson F. K., Focht D. D. Bacterial metabolism of hydroxylated biphenyls. Appl Environ Microbiol. 1989 Apr;55(4):946–952. doi: 10.1128/aem.55.4.946-952.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klecka G. M., Gibson D. T. Metabolism of Dibenzo-p-Dioxin and Chlorinated Dibenzo-p- Dioxins by a Beijerinckia Species. Appl Environ Microbiol. 1980 Feb;39(2):288–296. doi: 10.1128/aem.39.2.288-296.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klecka G. M., Gibson D. T. Metabolism of dibenzo[1,4]dioxan by a Pseudomonas species. Biochem J. 1979 Jun 15;180(3):639–645. doi: 10.1042/bj1800639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler H. P., Kohler-Staub D., Focht D. D. Degradation of 2-hydroxybiphenyl and 2,2'-dihydroxybiphenyl by Pseudomonas sp. strain HBP1. Appl Environ Microbiol. 1988 Nov;54(11):2683–2688. doi: 10.1128/aem.54.11.2683-2688.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massé R., Messier F., Péloquin L., Ayotte C., Sylvestre M. Microbial biodegradation of 4-chlorobiphenyl, a model compound of chlorinated biphenyls. Appl Environ Microbiol. 1984 May;47(5):947–951. doi: 10.1128/aem.47.5.947-951.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamsuzzaman K. M., Barnsley E. A. The regulation of naphthalene metabolism in pseudomonads. Biochem Biophys Res Commun. 1974 Sep 23;60(2):582–589. doi: 10.1016/0006-291x(74)90280-0. [DOI] [PubMed] [Google Scholar]
- Spector T. Refinement of the coomassie blue method of protein quantitation. A simple and linear spectrophotometric assay for less than or equal to 0.5 to 50 microgram of protein. Anal Biochem. 1978 May;86(1):142–146. doi: 10.1016/0003-2697(78)90327-5. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y. The Oxidation of Aromatic Compounds by Fluorescent Pseudomonads. J Bacteriol. 1948 Apr;55(4):477–494. doi: 10.1128/jb.55.4.477-494.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strubel V., Rast H. G., Fietz W., Knackmuss H. J., Engesser K. H. Enrichment of dibenzofuran utilizing bacteria with high co-metabolic potential towards dibenzodioxin and other anellated aromatics. FEMS Microbiol Lett. 1989 Apr;49(2-3):233–238. doi: 10.1016/0378-1097(89)90044-x. [DOI] [PubMed] [Google Scholar]
- Tomasek P. H., Crawford R. L. Initial reactions of xanthone biodegradation by an Arthrobacter sp. J Bacteriol. 1986 Sep;167(3):818–827. doi: 10.1128/jb.167.3.818-827.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelis M. L., Palleroni N. J., Stanier R. Y. The metabolism of aromatic acids by Pseudomonas testosteroni and P. acidovorans. Arch Mikrobiol. 1967;59(1):302–314. doi: 10.1007/BF00406344. [DOI] [PubMed] [Google Scholar]


