Abstract
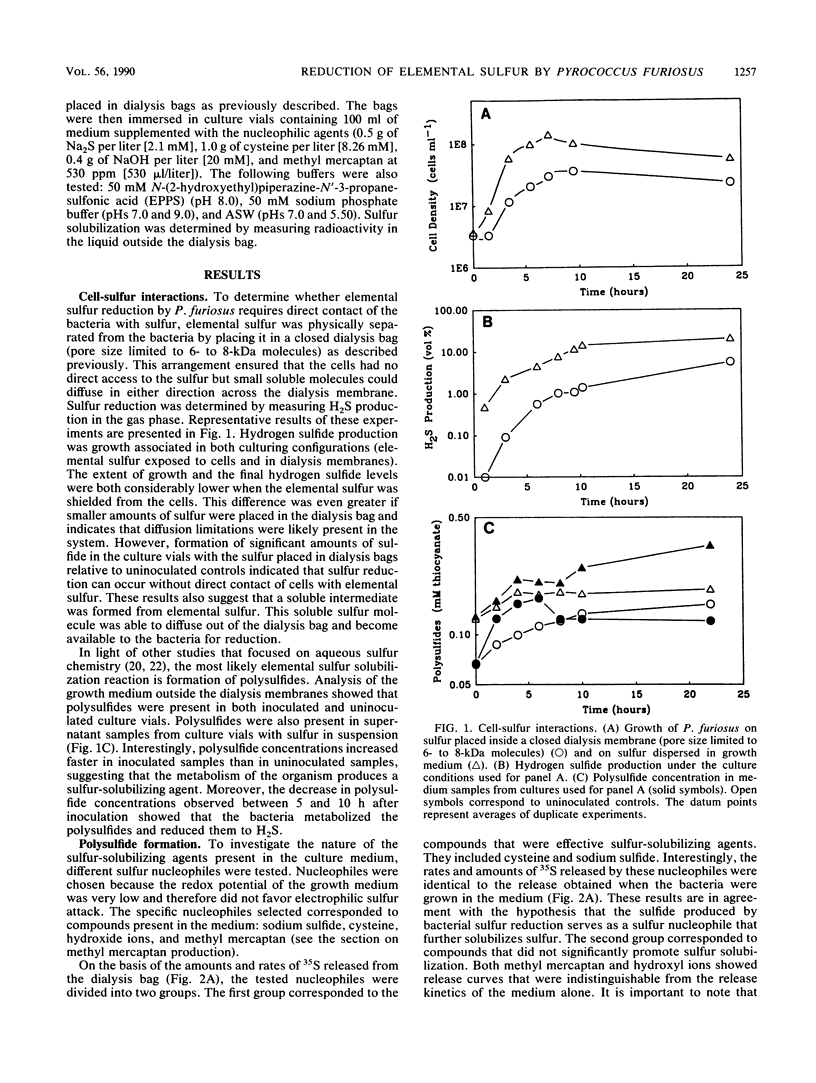
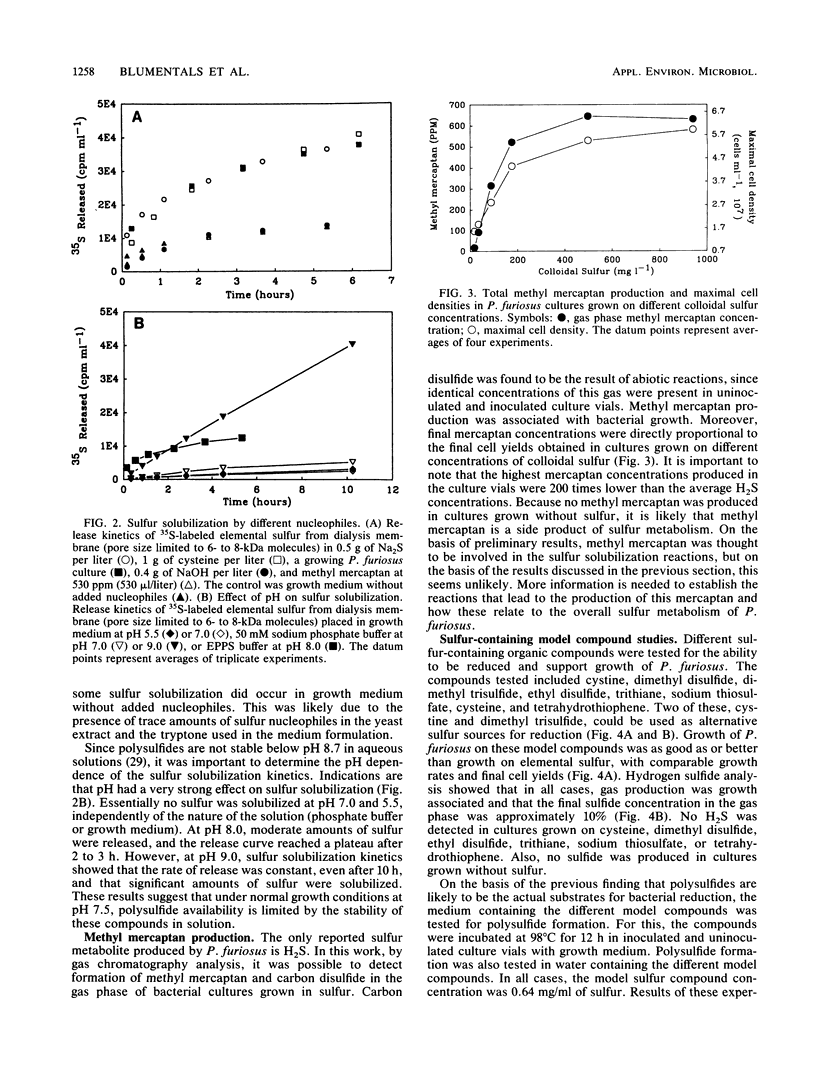
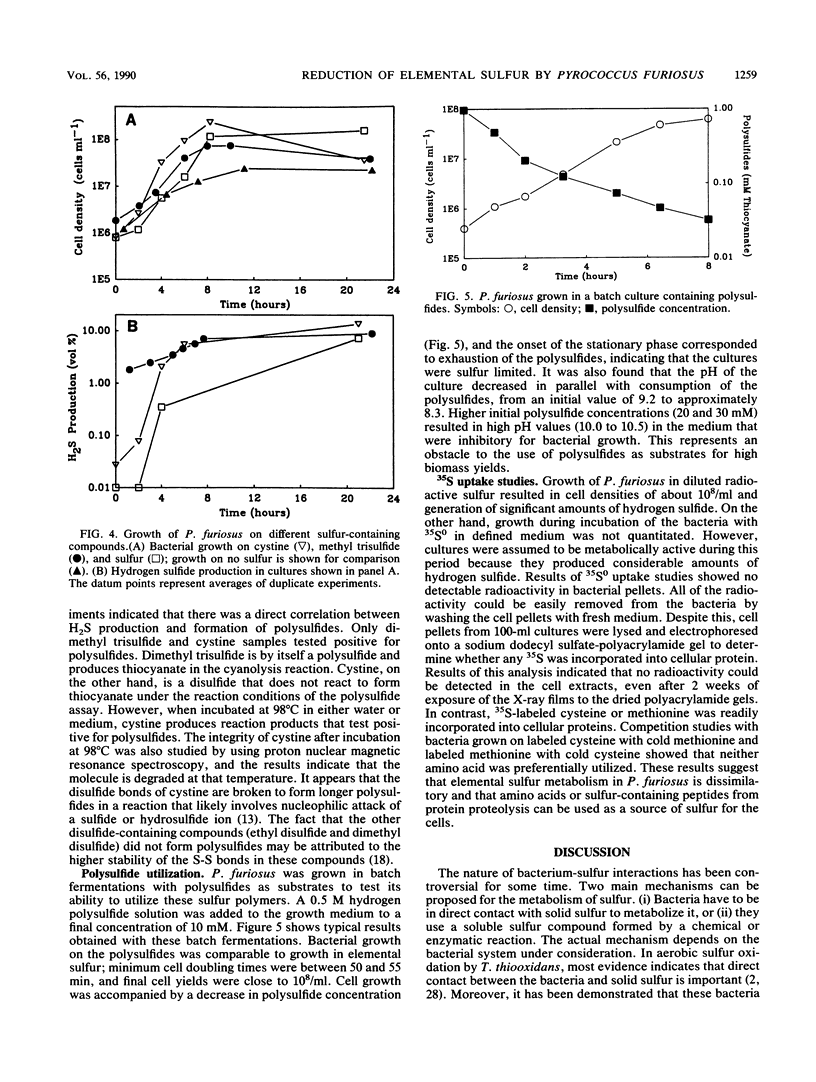
Polysulfides formed through the breakdown of elemental sulfur or other sulfur compounds were found to be reduced to H2S by the hyperthermophilic archaebacterium Pyrococcus furiosus during growth. Metabolism of polysulfides by the organism was dissimilatory, as no incorporation of 35S-labeled elemental sulfur was detected. However, [35S]cysteine and [35S]methionine were incorporated into cellular protein. Contact between the organism and elemental sulfur is not necessary for metabolism. The sulfide generated from metabolic reduction of polysulfides dissociates to a strong nucleophile, HS−, which in turn opens up the S8 elemental sulfur ring. In addition to H2S, P. furiosus cultures produced methyl mercaptan in a growth-associated fashion.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aono S., Bryant F. O., Adams M. W. A novel and remarkably thermostable ferredoxin from the hyperthermophilic archaebacterium Pyrococcus furiosus. J Bacteriol. 1989 Jun;171(6):3433–3439. doi: 10.1128/jb.171.6.3433-3439.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldensperger J., Guarraia L. J., Humphreys W. J. Scanning electron microscopy of thiobacilli grown on colloïdal sulfur. Arch Microbiol. 1974;99(4):323–329. doi: 10.1007/BF00696246. [DOI] [PubMed] [Google Scholar]
- Belkin S., Wirsen C. O., Jannasch H. W. Biological and abiological sulfur reduction at high temperatures. Appl Environ Microbiol. 1985 May;49(5):1057–1061. doi: 10.1128/aem.49.5.1057-1061.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant F. O., Adams M. W. Characterization of hydrogenase from the hyperthermophilic archaebacterium, Pyrococcus furiosus. J Biol Chem. 1989 Mar 25;264(9):5070–5079. [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES G. E., STARKEY R. L. Surface-active substances produced by Thiobacillus thiooxidans. J Bacteriol. 1961 Nov;82:788–789. doi: 10.1128/jb.82.5.788-789.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly D. P. Physiology of the thiobacilli: elucidating the sulphur oxidation pathway. Microbiol Sci. 1985;2(4):105–109. [PubMed] [Google Scholar]
- Suzuki I. Oxidation of elemental sulfur by an enzyme system of Thiobacillus thiooxidans. Biochim Biophys Acta. 1965 Jul 8;104(2):359–371. doi: 10.1016/0304-4165(65)90341-7. [DOI] [PubMed] [Google Scholar]


