Abstract
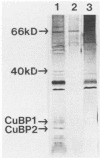
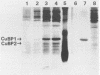
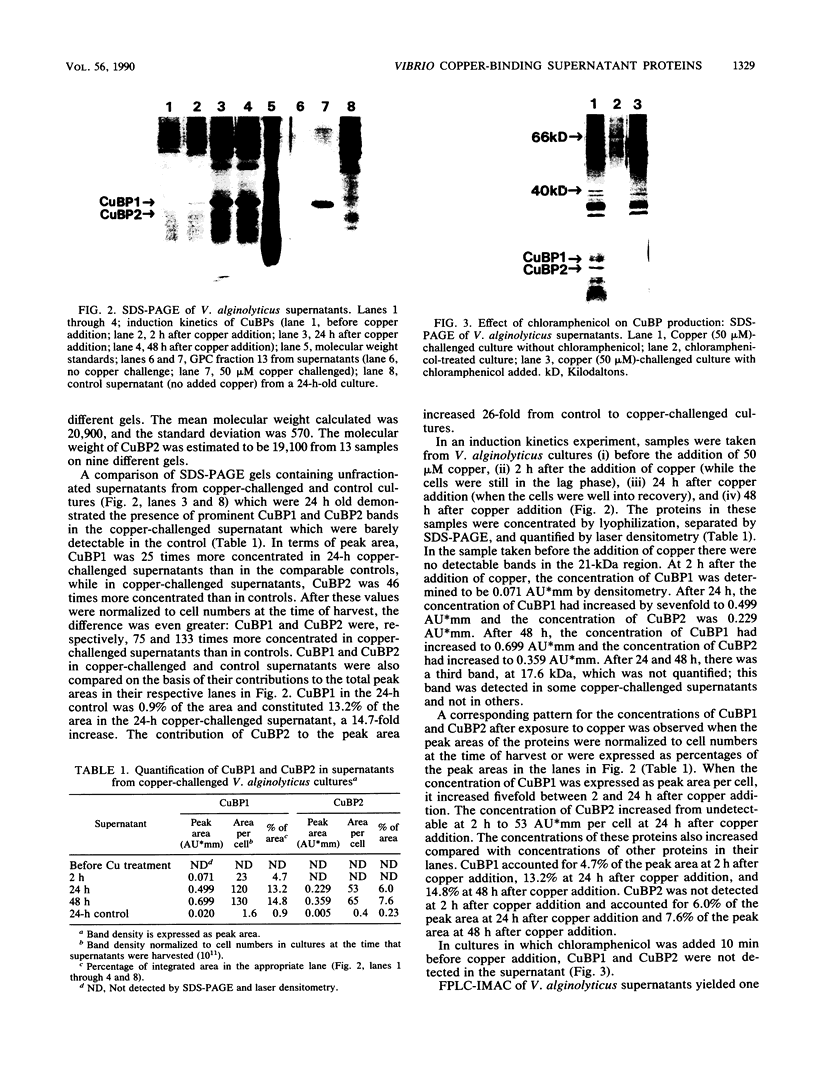
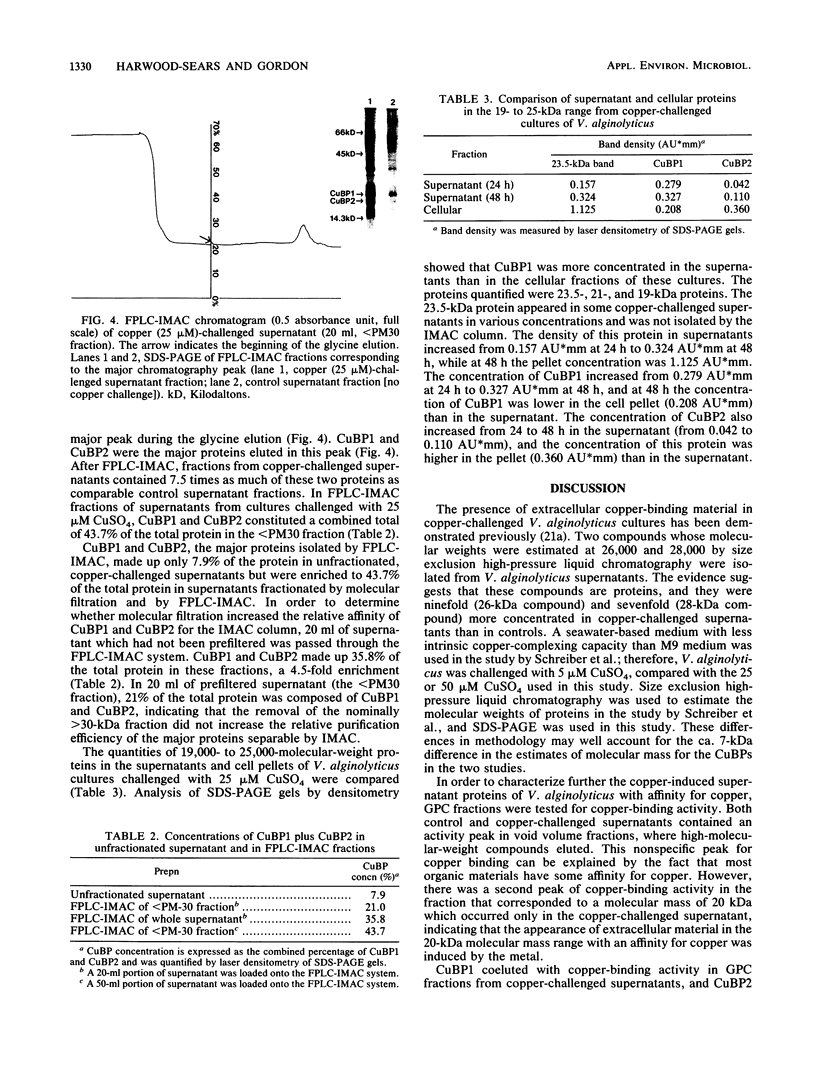
Growth of the marine bacterium Vibrio alginolyticus is temporarily inhibited by micromolar levels of copper. During the copper-induced lag phase, supernatant compounds which complex and detoxify copper are produced. In this study two copper-inducible supernatant proteins having molecular masses of ca. 21 and 19 kilodaltons (CuBP1 and CuBP2) were identified; these proteins were, respectively, 25 and 46 times amplified in supernatants of copper-challenged cultures compared with controls. Experiments in which chloramphenicol was added to cultures indicated that there was de novo synthesis of these proteins in response to copper. When supernatants were separated by gel permeation chromatography, CuBP1 and CuBP2 coeluted with a copper-induced peak in copper-binding activity. CuBP1 and CuBP2 from whole supernatants were concentrated and partially purified by using a copper-charged immobilized metal ion affinity chromatography column, confirming the affinity of these proteins for copper. A comparison of cell pellets and supernatants demonstrated that CuBP1 was more concentrated in supernatants than in cells. Our data are consistent with a model for a novel mechanism of copper detoxification in which excretion of copper-binding protein is induced by copper.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Casida L. E. Relation to copper of N-1, a nonobligate bacterial predator. Appl Environ Microbiol. 1987 Jul;53(7):1515–1518. doi: 10.1128/aem.53.7.1515-1518.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke S. E., Stuart J., Sanders-Loehr J. Induction of siderophore activity in Anabaena spp. and its moderation of copper toxicity. Appl Environ Microbiol. 1987 May;53(5):917–922. doi: 10.1128/aem.53.5.917-922.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erardi F. X., Failla M. L., Falkinham J. O., 3rd Plasmid-encoded copper resistance and precipitation by Mycobacterium scrofulaceum. Appl Environ Microbiol. 1987 Aug;53(8):1951–1954. doi: 10.1128/aem.53.8.1951-1954.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. S., Gerchakov S. M., Millero F. J. Effects of inorganic particles on metabolism by a periphytic marine bacterium. Appl Environ Microbiol. 1983 Feb;45(2):411–417. doi: 10.1128/aem.45.2.411-417.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higham D. P., Sadler P. J., Scawen M. D. Cadmium-Resistant Pseudomonas putida Synthesizes Novel Cadmium Proteins. Science. 1984 Sep 7;225(4666):1043–1046. doi: 10.1126/science.225.4666.1043. [DOI] [PubMed] [Google Scholar]
- Karin M., Najarian R., Haslinger A., Valenzuela P., Welch J., Fogel S. Primary structure and transcription of an amplified genetic locus: the CUP1 locus of yeast. Proc Natl Acad Sci U S A. 1984 Jan;81(2):337–341. doi: 10.1073/pnas.81.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mittelman M. W., Geesey G. G. Copper-binding characteristics of exopolymers from a freshwater-sediment bacterium. Appl Environ Microbiol. 1985 Apr;49(4):846–851. doi: 10.1128/aem.49.4.846-851.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]