Abstract
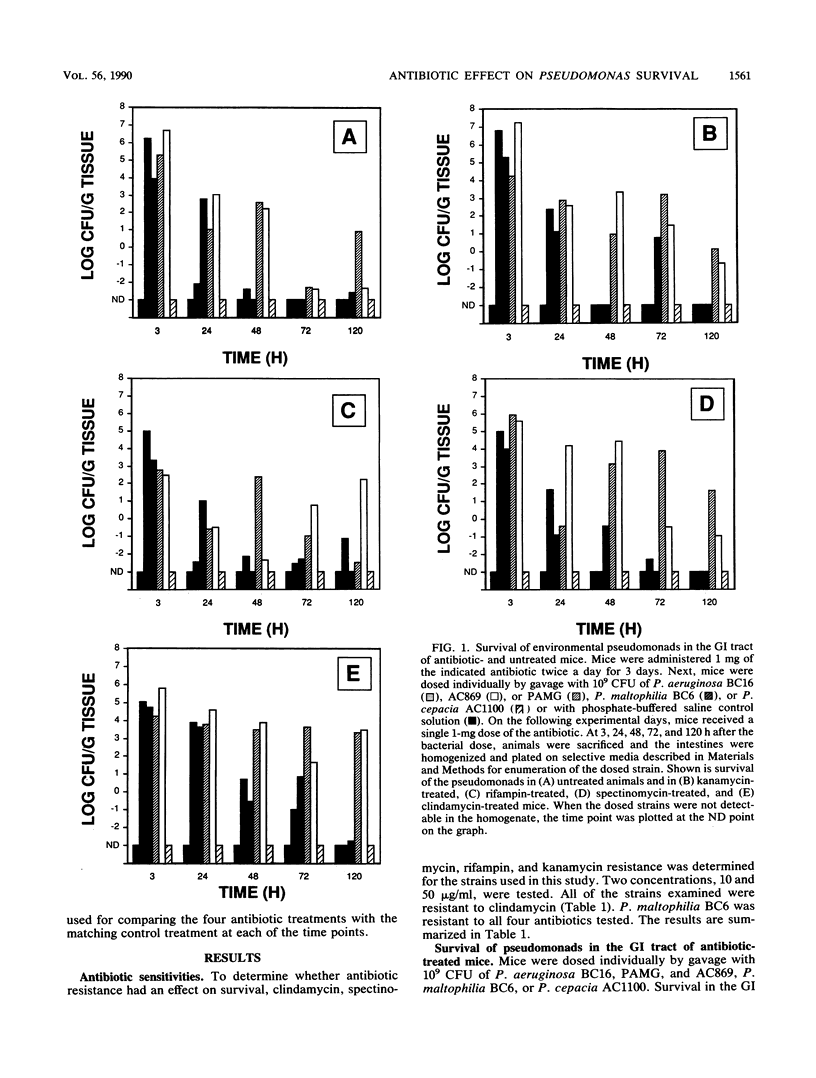
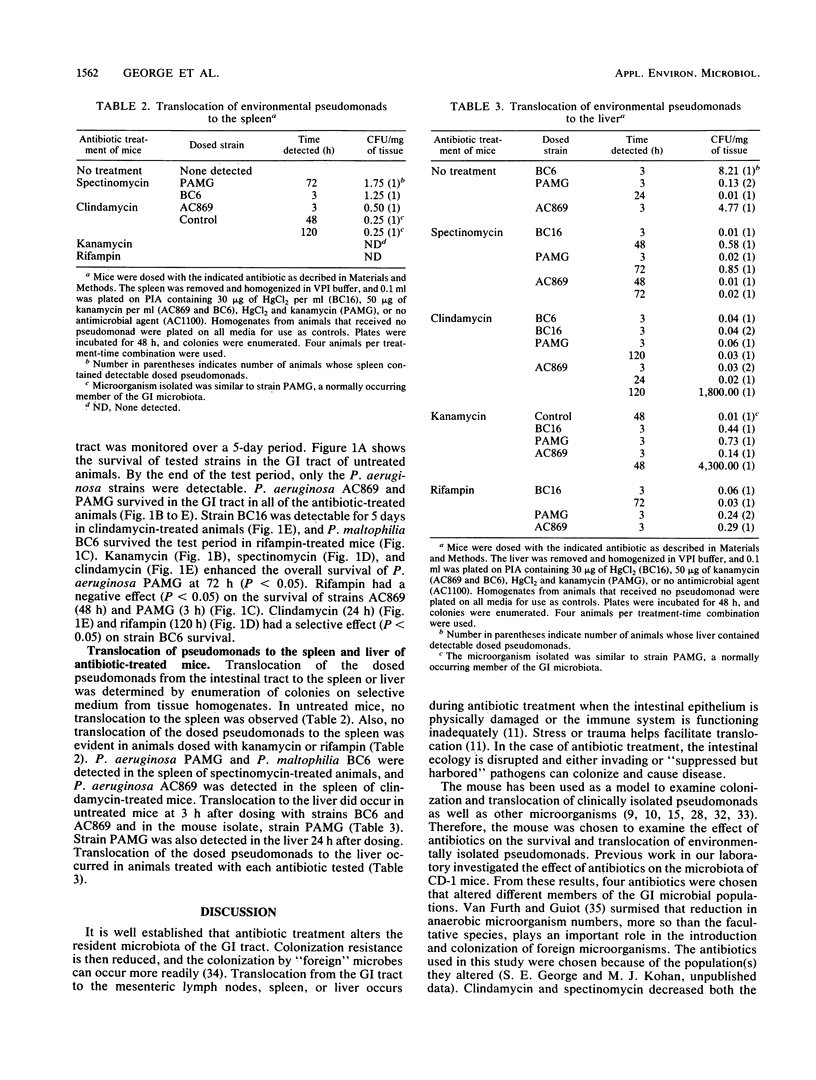
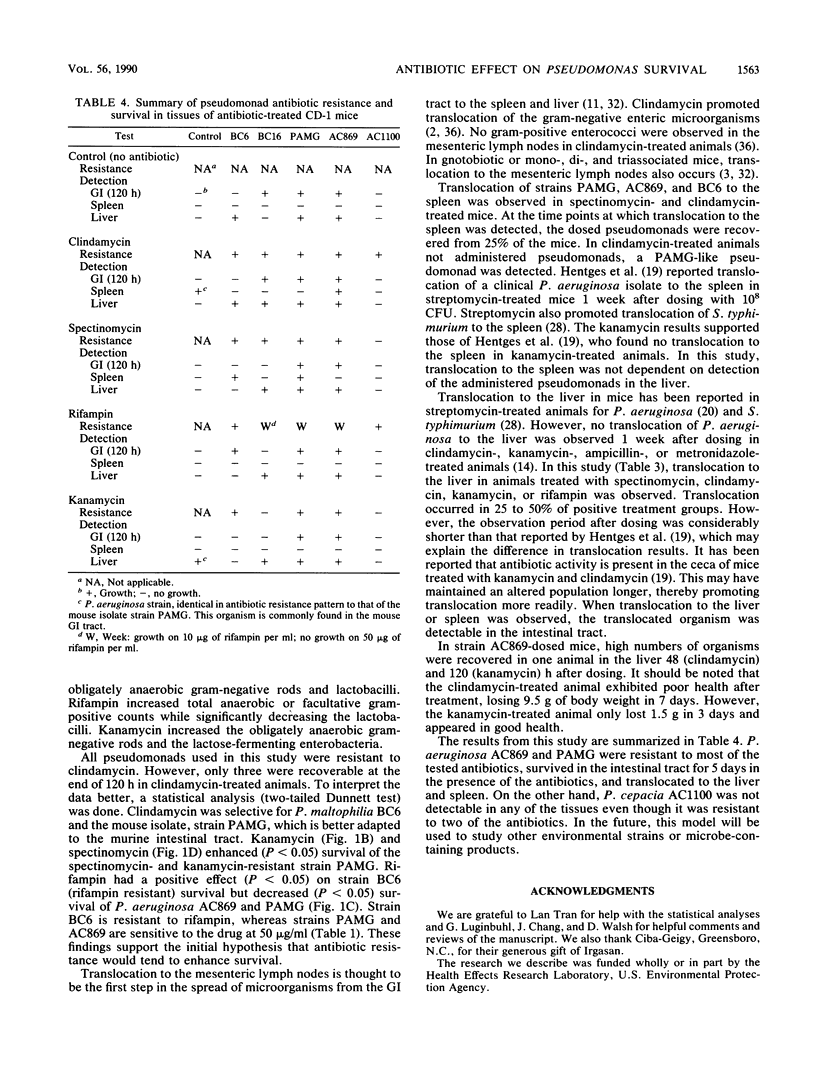
The environmental release of microorganisms has prompted the investigation of potential health effects associated with their release. In this study, survival and translocation to the spleen and liver of several environmental Pseudomonas spp. were investigated in antibiotic-treated mice. Pseudomonas aeruginosa BC16 and P. maltophilia BC6, isolated from a commercial product for polychlorinated biphenyl degradation; P. aeruginosa AC869, a 3,5-dichlorobenzoate degrader; and P. cepacia AC1100, an organism that metabolizes 2,4,5-trichlorophenoxyacetic acid were examined for their survival capabilities in the intestines of mice dosed with clindamycin, kanamycin, rifampin, or spectinomycin. A mouse intestinal isolate, strain PAMG, was included in the study. Following antibiotic pretreatment (1 mg twice daily for 3 days), mice were dosed by gavage with 10(9) CFU of each Pseudomonas strain. At the end of the 5-day test period, strains AC869 and PAMG survived in kanamycin-, rifampin-, spectinomycin-, and clindamycin-treated animals. A statistically significant (P less than 0.05) increase in survival of strain PAMG was observed in clindamycin-, kanamycin-, and spectinomycin-treated mice for the test period. Treatment with clindamycin or rifampin increased (P less than 0.05) survival of strain BC6, an organism resistant to both antibiotics. However, strain BC6 was detected only in rifampin-treated mice at the end of the 5-day test period. Strain BC16, a clindamycin-resistant strain, was detected in clindamycin-treated mice and the untreated control animals. Rifampin had a negative effect (P less than 0.05) on strain AC869 and PAMG survival. Translocation to the spleen was observed in spectinomycin- and clindamycin-treated mice but was not detected in kanamycin- or rifampin-treated animals.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartlett J. G., Moon N., Chang T. W., Taylor N., Onderdonk A. B. Role of Clostridium difficile in antibiotic-associated pseudomembranous colitis. Gastroenterology. 1978 Nov;75(5):778–782. [PubMed] [Google Scholar]
- Berg R. D., Garlington A. W. Translocation of certain indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes and other organs in a gnotobiotic mouse model. Infect Immun. 1979 Feb;23(2):403–411. doi: 10.1128/iai.23.2.403-411.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg R. D. Promotion of the translocation of enteric bacteria from the gastrointestinal tracts of mice by oral treatment with penicillin, clindamycin, or metronidazole. Infect Immun. 1981 Sep;33(3):854–861. doi: 10.1128/iai.33.3.854-861.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodey G. P., Rodriguez V., Chang H. Y., Narboni Fever and infection in leukemic patients: a study of 494 consecutive patients. Cancer. 1978 Apr;41(4):1610–1622. doi: 10.1002/1097-0142(197804)41:4<1610::aid-cncr2820410452>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Chatterjee D. K., Chakrabarty A. M. Genetic rearrangements in plasmids specifying total degradation of chlorinated benzoic acids. Mol Gen Genet. 1982;188(2):279–285. doi: 10.1007/BF00332688. [DOI] [PubMed] [Google Scholar]
- Chatterjee D. K., Kellogg S. T., Hamada S., Chakrabarty A. M. Plasmid specifying total degradation of 3-chlorobenzoate by a modified ortho pathway. J Bacteriol. 1981 May;146(2):639–646. doi: 10.1128/jb.146.2.639-646.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow A. W., Taylor P. R., Yoshikawa T. T., Guze L. B. A nosocomial outbreak of infections due to multiply resistant Proteus mirabilis: role of intestinal colonization as a major reservoir. J Infect Dis. 1979 Jun;139(6):621–627. doi: 10.1093/infdis/139.6.621. [DOI] [PubMed] [Google Scholar]
- Clark J. D. Influence of antibiotics or certain intestinal bacteria on orally administered Candida albicans in germ-free and conventional mice. Infect Immun. 1971 Dec;4(6):731–737. doi: 10.1128/iai.4.6.731-737.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitch E. A., Berg R. Bacterial translocation from the gut: a mechanism of infection. J Burn Care Rehabil. 1987 Nov-Dec;8(6):475–482. [PubMed] [Google Scholar]
- Freter R., Brickner H., Fekete J., Vickerman M. M., Carey K. E. Survival and implantation of Escherichia coli in the intestinal tract. Infect Immun. 1983 Feb;39(2):686–703. doi: 10.1128/iai.39.2.686-703.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George S. E., Kohan M. J., Walsh D. B., Stead A. G., Claxton L. D. Polychlorinated biphenyl-degrading pseudomonads: survival in mouse intestines and competition with normal flora. J Toxicol Environ Health. 1989;26(1):19–37. doi: 10.1080/15287398909531231. [DOI] [PubMed] [Google Scholar]
- George S. E., Walsh D. B., Stead A. G., Claxton L. D. Effect of ampicillin-induced alterations in murine intestinal microbiota on the survival and competition of environmentally released pseudomonads. Fundam Appl Toxicol. 1989 Nov;13(4):670–680. doi: 10.1016/0272-0590(89)90325-4. [DOI] [PubMed] [Google Scholar]
- Guiot H. F., van der Meer J. W., van Furth R. Selective antimicrobial modulation of human microbial flora: infection prevention in patients with decreased host defense mechanisms by selective elimination of potentially pathogenic bacteria. J Infect Dis. 1981 May;143(5):644–654. doi: 10.1093/infdis/143.5.644. [DOI] [PubMed] [Google Scholar]
- Hentges D. J., Stein A. J., Casey S. W., Que J. U. Protective role of intestinal flora against infection with Pseudomonas aeruginosa in mice: influence of antibiotics on colonization resistance. Infect Immun. 1985 Jan;47(1):118–122. doi: 10.1128/iai.47.1.118-122.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzman R. S., Florman A. L., Podrid P. J., Simberkoff M. S., Toharsky B. Drug-associated diarrhoea as a potential reservoir for hospital infections. Lancet. 1974 Jun 15;1(7868):1195–1196. doi: 10.1016/s0140-6736(74)91004-6. [DOI] [PubMed] [Google Scholar]
- Kilbane J. J., Chatterjee D. K., Karns J. S., Kellogg S. T., Chakrabarty A. M. Biodegradation of 2,4,5-trichlorophenoxyacetic acid by a pure culture of Pseudomonas cepacia. Appl Environ Microbiol. 1982 Jul;44(1):72–78. doi: 10.1128/aem.44.1.72-78.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M. J., Montgomery S. O., Mahaffey W. R., Pritchard P. H. Biodegradation of trichloroethylene and involvement of an aromatic biodegradative pathway. Appl Environ Microbiol. 1987 May;53(5):949–954. doi: 10.1128/aem.53.5.949-954.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obukowicz M. G., Perlak F. J., Kusano-Kretzmer K., Mayer E. J., Bolten S. L., Watrud L. S. Tn5-mediated integration of the delta-endotoxin gene from Bacillus thuringiensis into the chromosome of root-colonizing pseudomonads. J Bacteriol. 1986 Nov;168(2):982–989. doi: 10.1128/jb.168.2.982-989.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que J. U., Hentges D. J. Effect of streptomycin administration on colonization resistance to Salmonella typhimurium in mice. Infect Immun. 1985 Apr;48(1):169–174. doi: 10.1128/iai.48.1.169-174.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimpff S. C. Infection prevention during profound granulocytopenia. New approaches to alimentary canal microbial suppression. Ann Intern Med. 1980 Aug;93(2):358–361. doi: 10.7326/0003-4819-93-2-358. [DOI] [PubMed] [Google Scholar]
- Selden R., Lee S., Wang W. L., Bennett J. V., Eickhoff T. C. Nosocomial klebsiella infections: intestinal colonization as a reservoir. Ann Intern Med. 1971 May;74(5):657–664. doi: 10.7326/0003-4819-74-5-657. [DOI] [PubMed] [Google Scholar]
- Steffen E. K., Berg R. D. Relationship between cecal population levels of indigenous bacteria and translocation to the mesenteric lymph nodes. Infect Immun. 1983 Mar;39(3):1252–1259. doi: 10.1128/iai.39.3.1252-1259.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells C. L., Jechorek R. P., Maddaus M. A., Simmons R. L. Effects of clindamycin and metronidazole on the intestinal colonization and translocation of enterococci in mice. Antimicrob Agents Chemother. 1988 Dec;32(12):1769–1775. doi: 10.1128/aac.32.12.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Furth R., Guiot H. F. Modulation of the host flora. Eur J Clin Microbiol Infect Dis. 1989 Jan;8(1):1–7. doi: 10.1007/BF01964112. [DOI] [PubMed] [Google Scholar]
- van der Waaij D., Berghuis-de Vries J. M., Lekkerkerk Lekkerkerk-v Colonization resistance of the digestive tract in conventional and antibiotic-treated mice. J Hyg (Lond) 1971 Sep;69(3):405–411. doi: 10.1017/s0022172400021653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Waaij D., de Vries-Hospers H. G., Welling G. W. The influence of antibiotics on gut colonization. J Antimicrob Chemother. 1986 Oct;18 (Suppl 100):155–158. doi: 10.1093/jac/18.supplement_c.155. [DOI] [PubMed] [Google Scholar]


