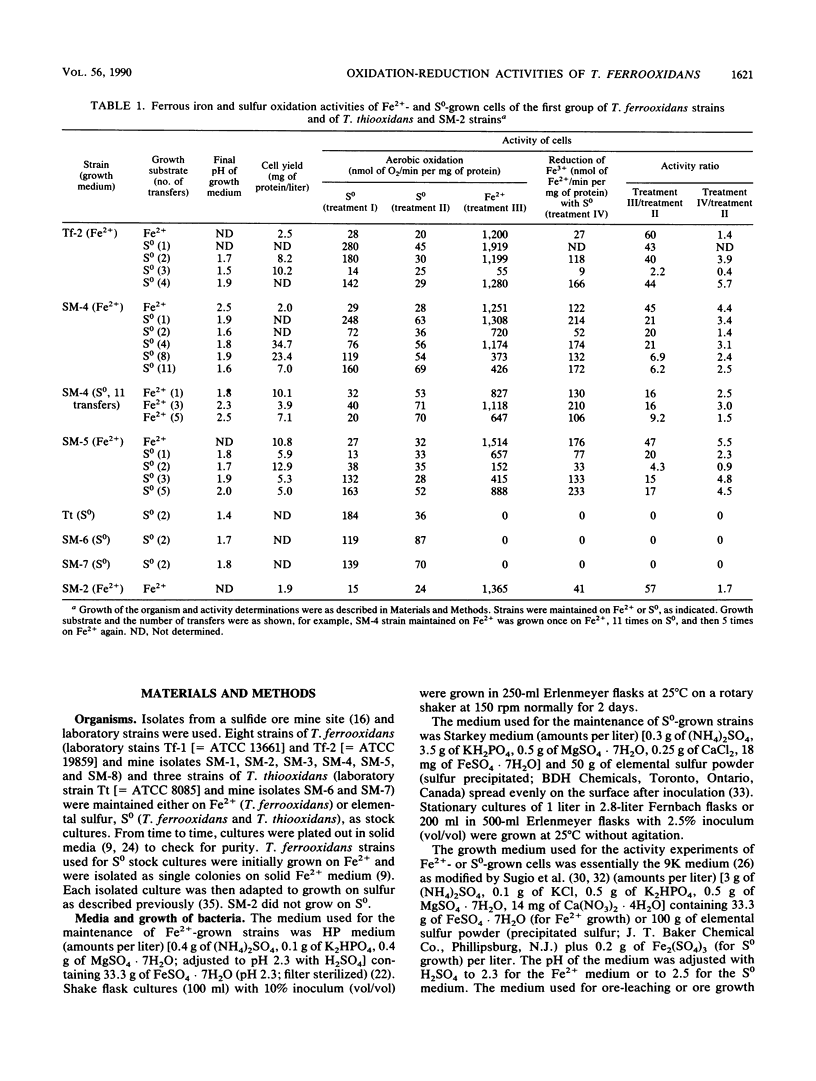
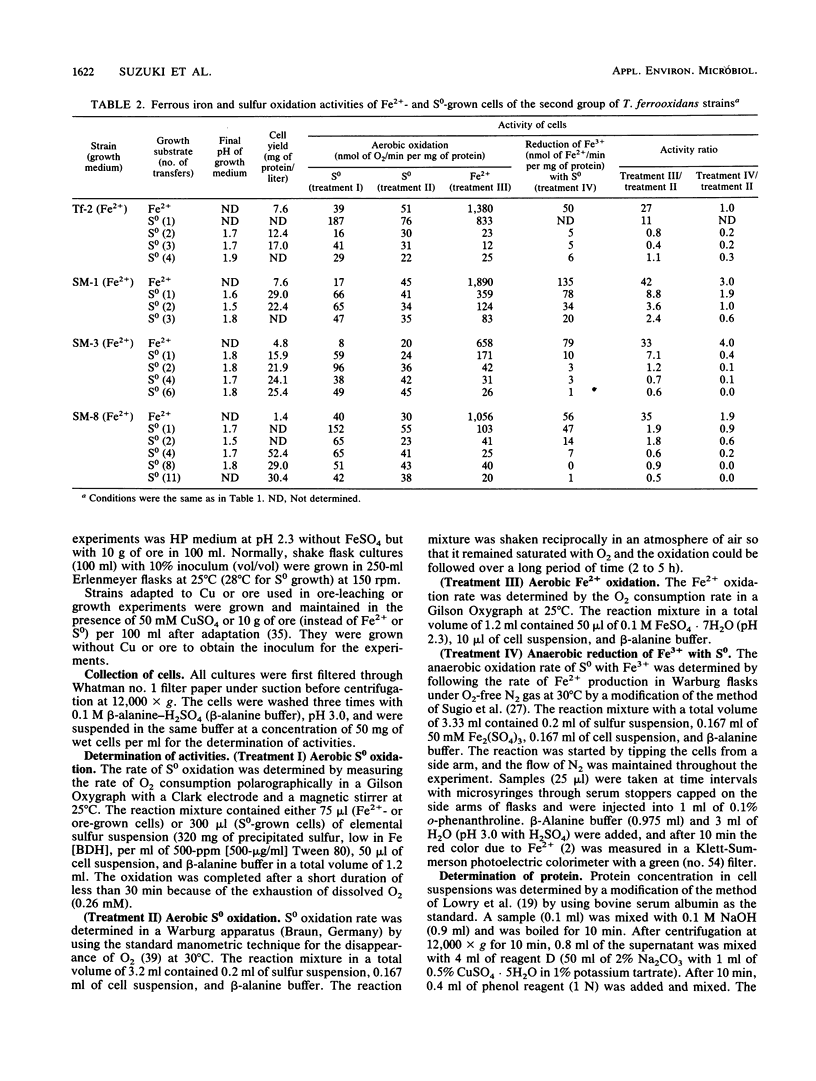
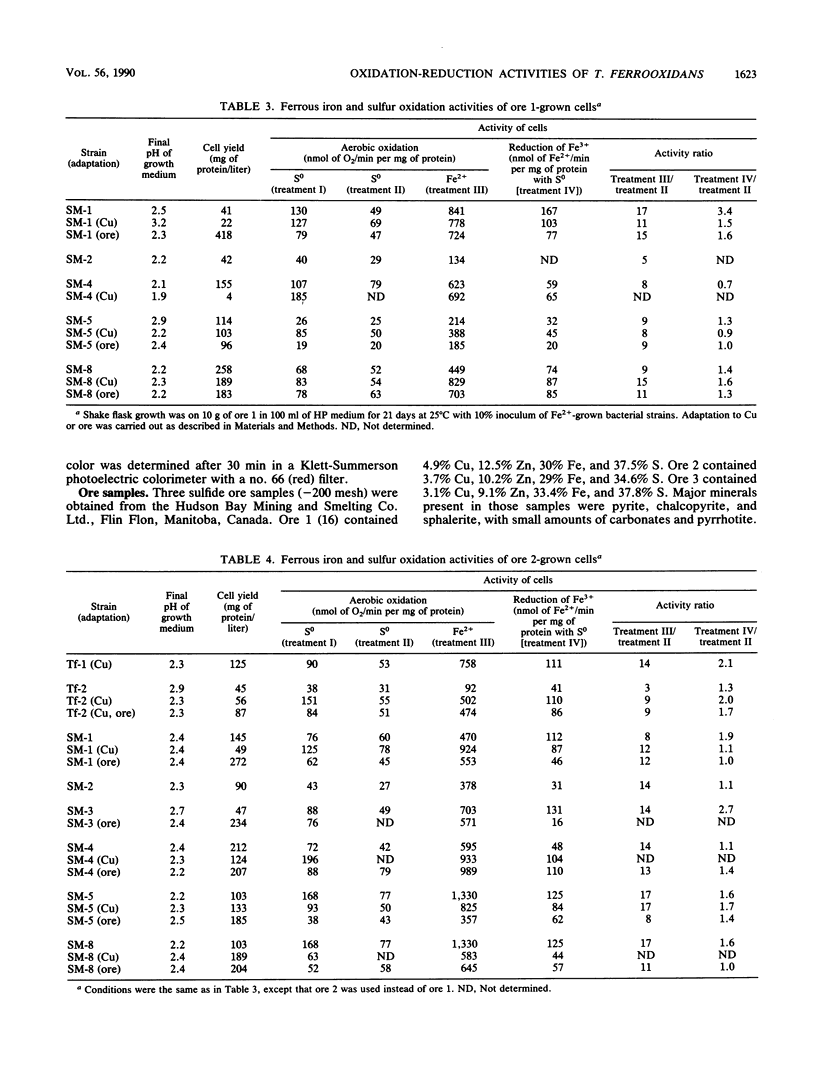
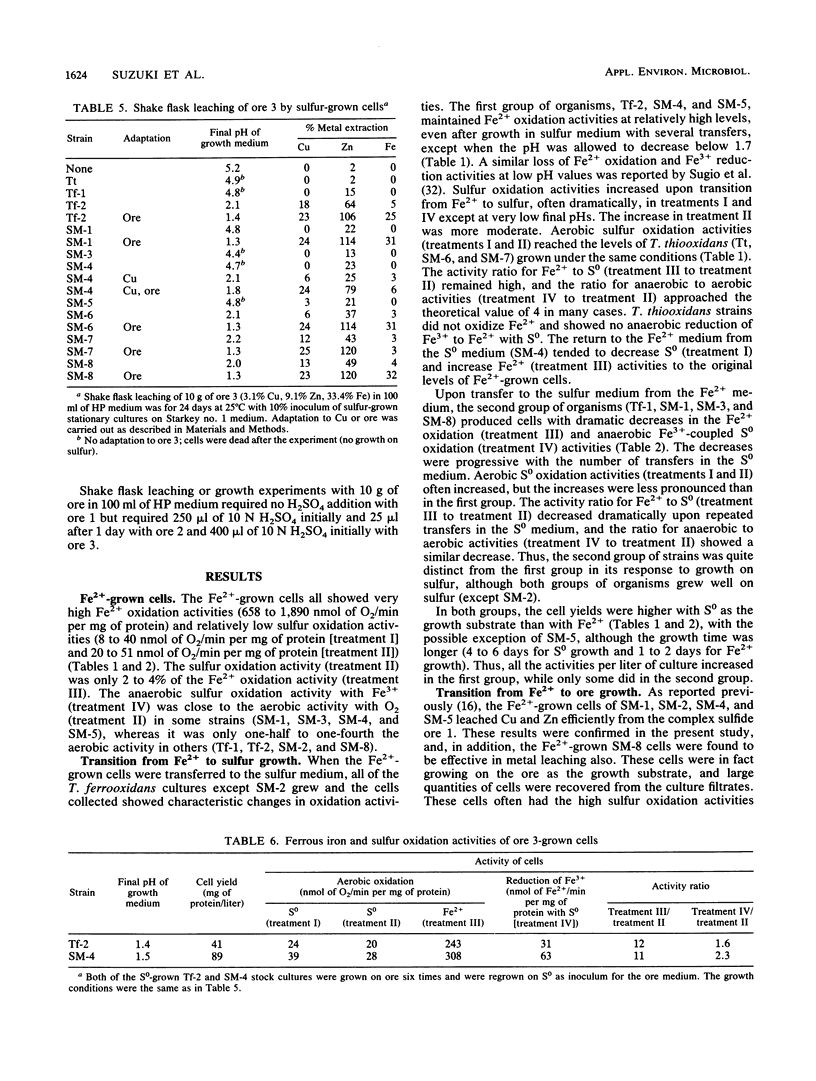
Abstract
Eight strains of Thiobacillus ferrooxidans (laboratory strains Tf-1 [= ATCC 13661] and Tf-2 [= ATCC 19859] and mine isolates SM-1, SM-2, SM-3, SM-4, SM-5, and SM-8) and three strains of Thiobacillus thiooxidans (laboratory strain Tt [= ATCC 8085] and mine isolates SM-6 and SM-7) were grown on ferrous iron (Fe2+), elemental sulfur (S0), or sulfide ore (Fe, Cu, and Zn). The cells were studied for their aerobic Fe2+ - and S0-oxidizing activities (O2 consumption) and anaerobic S0-oxidizing activity with ferric iron (Fe3+) (Fe2+ formation). Fe2+-grown T. ferrooxidans cells oxidized S0 aerobically at a rate of 2 to 4% of the Fe2+ oxidation rate. The rate of anaerobic S0 oxidation with Fe3+ was equal to the aerobic oxidation rate in SM-1, SM-3, SM-4, and SM-5, but was only one-half or less that in Tf-1, Tf-2, SM-2, and SM-8. Transition from growth on Fe2+ to that on S0 produced cells with relatively undiminished Fe2+ oxidation activities and increased S0 oxidation (both aerobic and anaerobic) activities in Tf-2, SM-4, and SM-5, whereas it produced cells with dramatically reduced Fe2+ oxidation and anaerobic S0 oxidation activities in Tf-1, SM-1, SM-2, SM-3, and SM-8. Growth on ore 1 of metal-leaching Fe2+-grown strains and on ore 2 of all Fe2+-grown strains resulted in very high yields of cells with high Fe2+ and S0 oxidation (both aerobic and anaerobic) activities with similar ratios of various activities. Sulfur-grown Tf-2, SM-1, SM-4, SM-6, SM-7, and SM-8 cultures leached metals from ore 3, and Tf-2 and SM-4 cells recovered showed activity ratios similar to those of other ore-grown cells. It is concluded that all the T. ferrooxidans strains studied have the ability to produce cells with Fe2+ and S0 oxidation and Fe3+ reduction activities, but their levels are influenced by growth substrates and strain differences.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cobley J. G., Haddock B. A. The respiratory chain of Thiobacillus ferrooxidans: the reduction of cytochromes by Fe2+ and the preliminary characterization of rusticyanin a novel "blue" copper protein. FEBS Lett. 1975 Dec 1;60(1):29–33. doi: 10.1016/0014-5793(75)80411-x. [DOI] [PubMed] [Google Scholar]
- Espejo R. T., Escobar B., Jedlicki E., Uribe P., Badilla-Ohlbaum R. Oxidation of Ferrous Iron and Elemental Sulfur by Thiobacillus ferrooxidans. Appl Environ Microbiol. 1988 Jul;54(7):1694–1699. doi: 10.1128/aem.54.7.1694-1699.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison A. P., Jr The acidophilic thiobacilli and other acidophilic bacteria that share their habitat. Annu Rev Microbiol. 1984;38:265–292. doi: 10.1146/annurev.mi.38.100184.001405. [DOI] [PubMed] [Google Scholar]
- Ingledew W. J. Thiobacillus ferrooxidans. The bioenergetics of an acidophilic chemolithotroph. Biochim Biophys Acta. 1982 Nov 30;683(2):89–117. doi: 10.1016/0304-4173(82)90007-6. [DOI] [PubMed] [Google Scholar]
- Kai M., Yano T., Fukumori Y., Yamanaka T. Cytochrome oxidase of an acidophilic iron-oxidizing bacterium, Thiobacillus ferrooxidans, functions at pH 3.5. Biochem Biophys Res Commun. 1989 Apr 28;160(2):839–843. doi: 10.1016/0006-291x(89)92510-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Landesman J., Duncan D. W., Walden C. C. Oxidation of inorganic sulfur compounds by washed cell suspensions of Thiobacillus ferrooxidans. Can J Microbiol. 1966 Oct;12(5):957–964. doi: 10.1139/m66-129. [DOI] [PubMed] [Google Scholar]
- Lizama H. M., Suzuki I. Rate Equations and Kinetic Parameters of the Reactions Involved in Pyrite Oxidation by Thiobacillus ferrooxidans. Appl Environ Microbiol. 1989 Nov;55(11):2918–2923. doi: 10.1128/aem.55.11.2918-2923.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren D. G., Silver M. Ore leaching by bacteria. Annu Rev Microbiol. 1980;34:263–283. doi: 10.1146/annurev.mi.34.100180.001403. [DOI] [PubMed] [Google Scholar]
- Margalith P., Silver M., Lundgren D. G. Sulfur oxidation by the iron bacterium Ferrobacillus ferrooxidans. J Bacteriol. 1966 Dec;92(6):1706–1709. doi: 10.1128/jb.92.6.1706-1709.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILVERMAN M. P., LUNDGREN D. G. Studies on the chemoautotrophic iron bacterium Ferrobacillus ferrooxidans. II. Manometric studies. J Bacteriol. 1959 Sep;78:326–331. doi: 10.1128/jb.78.3.326-331.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A., Fukumori Y., Yano T., Kai M., Yamanaka T. Thiobacillus ferrooxidans cytochrome c-552: purification and some of its molecular features. Biochim Biophys Acta. 1989 Sep 28;976(2-3):129–134. doi: 10.1016/s0005-2728(89)80221-x. [DOI] [PubMed] [Google Scholar]
- Schrader J. A., Holmes D. S. Phenotypic switching of Thiobacillus ferrooxidans. J Bacteriol. 1988 Sep;170(9):3915–3923. doi: 10.1128/jb.170.9.3915-3923.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver M., Lundgren D. G. Sulfur-oxidizing enzyme of Ferrobacillus ferrooxidans (Thiobacillus ferrooxidans). Can J Biochem. 1968 May;46(5):457–461. doi: 10.1139/o68-069. [DOI] [PubMed] [Google Scholar]
- Sugio T., Domatsu C., Munakata O., Tano T., Imai K. Role of a Ferric Ion-Reducing System in Sulfur Oxidation of Thiobacillus ferrooxidans. Appl Environ Microbiol. 1985 Jun;49(6):1401–1406. doi: 10.1128/aem.49.6.1401-1406.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugio T., Katagiri T., Moriyama M., Zhèn Y. L., Inagaki K., Tano T. Existence of a new type of sulfite oxidase which utilizes ferric ions as an electron acceptor in Thiobacillus ferrooxidans. Appl Environ Microbiol. 1988 Jan;54(1):153–157. doi: 10.1128/aem.54.1.153-157.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugio T., Mizunashi W., Inagaki K., Tano T. Purification and some properties of sulfur:ferric ion oxidoreductase from Thiobacillus ferrooxidans. J Bacteriol. 1987 Nov;169(11):4916–4922. doi: 10.1128/jb.169.11.4916-4922.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugio Tsuyoshi, Wada Kimihito, Mori Manami, Inagaki Kenji, Tano Tatsuo. Synthesis of an Iron-Oxidizing System during Growth of Thiobacillus ferrooxidans on Sulfur-Basal Salts Medium. Appl Environ Microbiol. 1988 Jan;54(1):150–152. doi: 10.1128/aem.54.1.150-152.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki I. Mechanisms of inorganic oxidation and energy coupling. Annu Rev Microbiol. 1974;28(0):85–101. doi: 10.1146/annurev.mi.28.100174.000505. [DOI] [PubMed] [Google Scholar]
- Suzuki I. Oxidation of elemental sulfur by an enzyme system of Thiobacillus thiooxidans. Biochim Biophys Acta. 1965 Jul 8;104(2):359–371. doi: 10.1016/0304-4165(65)90341-7. [DOI] [PubMed] [Google Scholar]
- Tuovinen O. H., Kelly D. P. Biology of Thiobacillus ferrooxidans in relation to the microbiological leaching of sulphide ores. Z Allg Mikrobiol. 1972;12(4):311–346. doi: 10.1002/jobm.3630120406. [DOI] [PubMed] [Google Scholar]
- Tuovinen O. H., Kelly D. P. Studies on the growth of Thiobacillus ferrooxidans. V. Factors affecting growth in liquid culture and development of colonies on solid media containing inorganic sulphur compounds. Arch Microbiol. 1974 Jul 22;98(4):351–364. doi: 10.1007/BF00425295. [DOI] [PubMed] [Google Scholar]


