Abstract
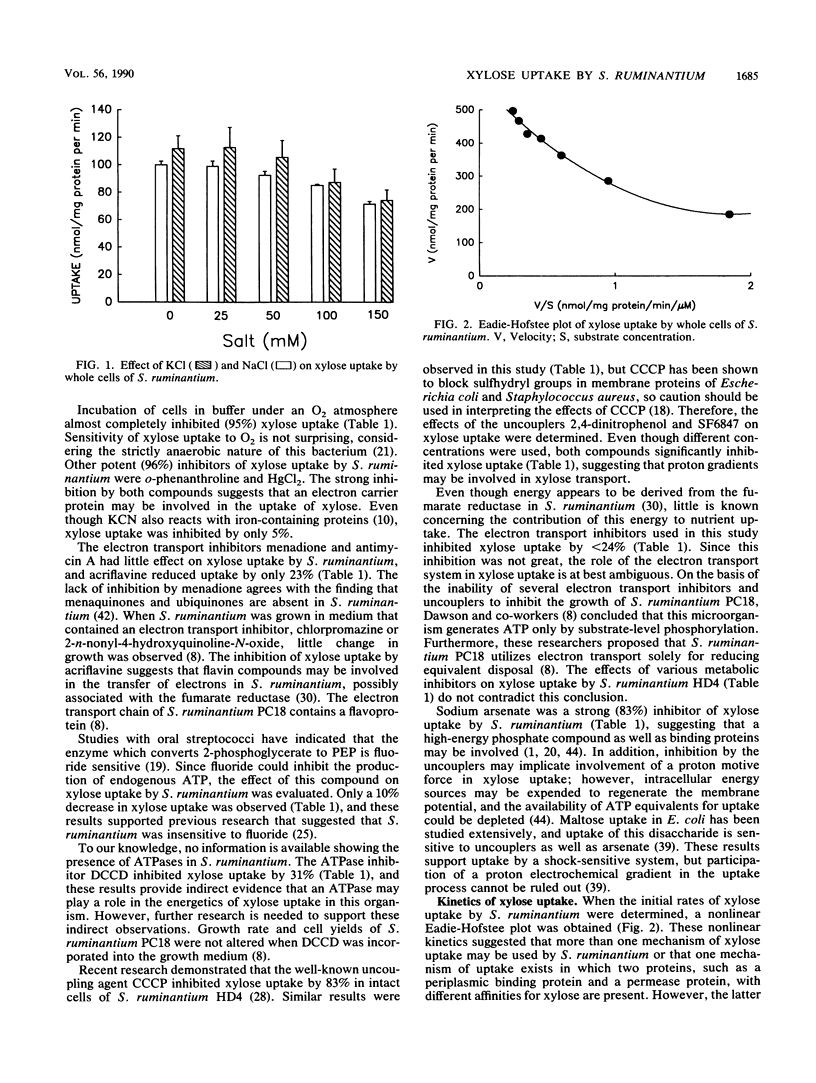
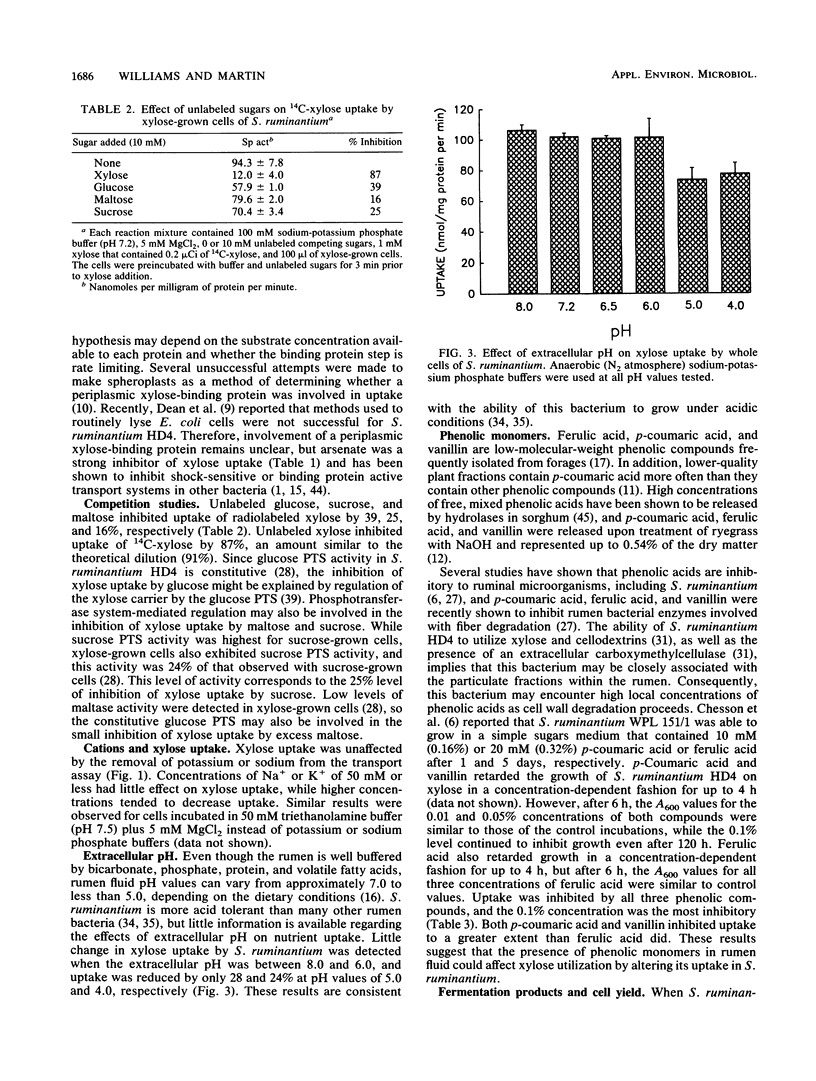
Selenomonas ruminantium HD4 does not use the phosphoenolpyruvate phosphotransferase system to transport xylose (S. A. Martin and J. B. Russell, J. Gen. Microbiol. 134:819-827, 1988). Xylose uptake by whole cells of S. ruminantium HD4 was inducible. Uptake was unaffected by monensin or lasalocid, while oxygen, o-phenanthroline, and HgCl2 were potent inhibitors. Menadione, antimycin A, and KCN had little effect on uptake, and acriflavine inhibited uptake by 23%. Sodium fluoride decreased xylose uptake by 10%, while N,N'-dicyclohexylcarbodiimide decreased uptake by 31%. Sodium arsenate was a strong inhibitor (83%), and these results suggest the involvement of a high-energy phosphate compound and possibly a binding protein in xylose uptake. The protonophores carbonyl cyanide m-chlorophenylhydrazone, 2,4-dinitrophenol, and SF6847 inhibited xylose uptake by 88, 82, and 43%, respectively. The cations Na+ and K+ did not stimulate xylose uptake. The kinetics of xylose uptake were nonlinear, and it appeared that more than one uptake mechanism may be involved or that two proteins (i.e., a binding protein and permease protein) with different affinities for xylose were present. Excess (10 mM) glucose, sucrose, or maltose decreased xylose uptake less than 40%. Uptake was unaffected at extracellular pH values between 6.0 and 8.0, while pH values of 5.0 and 4.0 decreased uptake 28 and 24%, respectively. The phenolic monomers p-coumaric acid and vanillin inhibited growth on xylose and xylose uptake more than ferulic acid did. The predominant end products resulting from the fermentation of xylose were lactate (7.5 mM), acetate (4.4 mM), and propionate (5.1 nM), and the Yxylose was 24.1 g/mol.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRYANT M. P. The characteristics of strains of Selenomonas isolated from bovine rumen contents. J Bacteriol. 1956 Aug;72(2):162–167. doi: 10.1128/jb.72.2.162-167.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger E. A., Heppel L. A. Different mechanisms of energy coupling for the shock-sensitive and shock-resistant amino acid permeases of Escherichia coli. J Biol Chem. 1974 Dec 25;249(24):7747–7755. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Caldwell D. R., Bryant M. P. Medium without rumen fluid for nonselective enumeration and isolation of rumen bacteria. Appl Microbiol. 1966 Sep;14(5):794–801. doi: 10.1128/am.14.5.794-801.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Wolin M. J. Effect of monensin and lasalocid-sodium on the growth of methanogenic and rumen saccharolytic bacteria. Appl Environ Microbiol. 1979 Jul;38(1):72–77. doi: 10.1128/aem.38.1.72-77.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesson A., Stewart C. S., Wallace R. J. Influence of plant phenolic acids on growth and cellulolytic activity of rumen bacteria. Appl Environ Microbiol. 1982 Sep;44(3):597–603. doi: 10.1128/aem.44.3.597-603.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson K. A., Preziosi M. C., Caldwell D. R. Some effects of uncouplers and inhibitors on growth and electron transport in rumen bacteria. J Bacteriol. 1979 Aug;139(2):384–392. doi: 10.1128/jb.139.2.384-392.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklund C. V., Glass T. L. Glucose uptake by the cellulolytic ruminal anaerobe Bacteroides succinogenes. J Bacteriol. 1987 Feb;169(2):500–506. doi: 10.1128/jb.169.2.500-506.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOBSON P. N. CONTINUOUS CULTURE OF SOME ANEROBIC AND FACULTATIVELY ANAEROBIC RUMEN BACTERIA. J Gen Microbiol. 1965 Feb;38:167–180. doi: 10.1099/00221287-38-2-167. [DOI] [PubMed] [Google Scholar]
- Hong J. S., Hunt A. G., Masters P. S., Lieberman M. A. Requirements of acetyl phosphate for the binding protein-dependent transport systems in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1213–1217. doi: 10.1073/pnas.76.3.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback H. R., Reeves J. P., Short S. A., Lombardi F. J. Mechanisms of active transport in isolated bacterial membrane vesicles. 18. The mechanism of action of carbonylcyanide m-chlorophenylhydrazone. Arch Biochem Biophys. 1974 Jan;160(1):215–222. doi: 10.1016/s0003-9861(74)80028-7. [DOI] [PubMed] [Google Scholar]
- Kanapka J. A., Hamilton I. R. Fluoride inhibition of enolase activity in vivo and its relationship to the inhibition of glucose-6-P formation in Streptococcus salivarius. Arch Biochem Biophys. 1971 Sep;146(1):167–174. doi: 10.1016/s0003-9861(71)80053-x. [DOI] [PubMed] [Google Scholar]
- Klein W. L., Boyer P. D. Energization of active transport by Escherichia coli. J Biol Chem. 1972 Nov 25;247(22):7257–7265. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Loesche W. J. Oxygen sensitivity of various anaerobic bacteria. Appl Microbiol. 1969 Nov;18(5):723–727. doi: 10.1128/am.18.5.723-727.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London J., Chace N. M. New pathway for the metabolism of pentitols. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4296–4300. doi: 10.1073/pnas.74.10.4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London J., Chace N. M. Pentitol metabolism in Lactobacillus casei. J Bacteriol. 1979 Dec;140(3):949–954. doi: 10.1128/jb.140.3.949-954.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. A., Akin D. E. Effect of phenolic monomers on the growth and beta-glucosidase activity of Bacteroides ruminicola and on the carboxymethylcellulase, beta-glucosidase, and xylanase activities of Bacteroides succinogenes. Appl Environ Microbiol. 1988 Dec;54(12):3019–3022. doi: 10.1128/aem.54.12.3019-3022.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. A., Russell J. B. Phosphoenolpyruvate-dependent phosphorylation of hexoses by ruminal bacteria: evidence for the phosphotransferase transport system. Appl Environ Microbiol. 1986 Dec;52(6):1348–1352. doi: 10.1128/aem.52.6.1348-1352.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. A., Russell J. B. Transport and phosphorylation of disaccharides by the ruminal bacterium Streptococcus bovis. Appl Environ Microbiol. 1987 Oct;53(10):2388–2393. doi: 10.1128/aem.53.10.2388-2393.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matin A., Veldkamp H. Physiological basis of the selective advantage of a Spirillum sp. in a carbon-limited environment. J Gen Microbiol. 1978 Apr;105(2):187–197. doi: 10.1099/00221287-105-2-187. [DOI] [PubMed] [Google Scholar]
- Melville S. B., Michel T. A., Macy J. M. Pathway and sites for energy conservation in the metabolism of glucose by Selenomonas ruminantium. J Bacteriol. 1988 Nov;170(11):5298–5304. doi: 10.1128/jb.170.11.5298-5304.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B., Baldwin R. L. Comparison of substrate affinities among several rumen bacteria: a possible determinant of rumen bacterial competition. Appl Environ Microbiol. 1979 Mar;37(3):531–536. doi: 10.1128/aem.37.3.531-536.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B., Baldwin R. L. Substrate preferences in rumen bacteria: evidence of catabolite regulatory mechanisms. Appl Environ Microbiol. 1978 Aug;36(2):319–329. doi: 10.1128/aem.36.2.319-329.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B., Dombrowski D. B. Effect of pH on the efficiency of growth by pure cultures of rumen bacteria in continuous culture. Appl Environ Microbiol. 1980 Mar;39(3):604–610. doi: 10.1128/aem.39.3.604-610.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B. Fermentation of cellodextrins by cellulolytic and noncellulolytic rumen bacteria. Appl Environ Microbiol. 1985 Mar;49(3):572–576. doi: 10.1128/aem.49.3.572-576.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B., Sharp W. M., Baldwin R. L. The effect of pH on maximum bacterial growth rate and its possible role as a determinant of bacterial competition in the rumen. J Anim Sci. 1979 Feb;48(2):251–255. doi: 10.2527/jas1979.482251x. [DOI] [PubMed] [Google Scholar]
- Russell J. B., Strobel H. J., Driessen A. J., Konings W. N. Sodium-dependent transport of neutral amino acids by whole cells and membrane vesicles of Streptococcus bovis, a ruminal bacterium. J Bacteriol. 1988 Aug;170(8):3531–3536. doi: 10.1128/jb.170.8.3531-3536.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B., Strobel H. J. Effect of ionophores on ruminal fermentation. Appl Environ Microbiol. 1989 Jan;55(1):1–6. doi: 10.1128/aem.55.1.1-6.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer D. M., Davis C. L., Bryant M. P. Ammonia saturation constants for predominant species of rumen bacteria. J Dairy Sci. 1980 Aug;63(8):1248–1263. doi: 10.3168/jds.S0022-0302(80)83076-1. [DOI] [PubMed] [Google Scholar]
- Wilson D. B. Source of energy for the Escherichia coli galactose transport systems induced by galactose. J Bacteriol. 1974 Nov;120(2):866–871. doi: 10.1128/jb.120.2.866-871.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


