Abstract
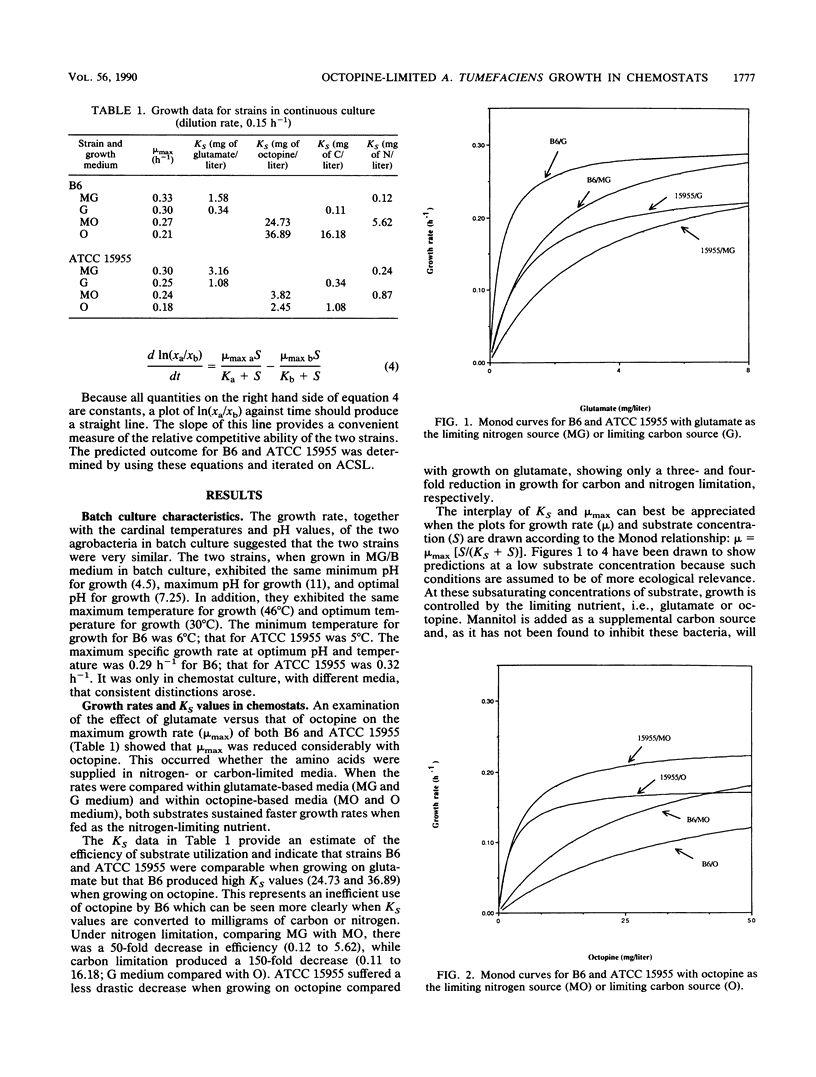
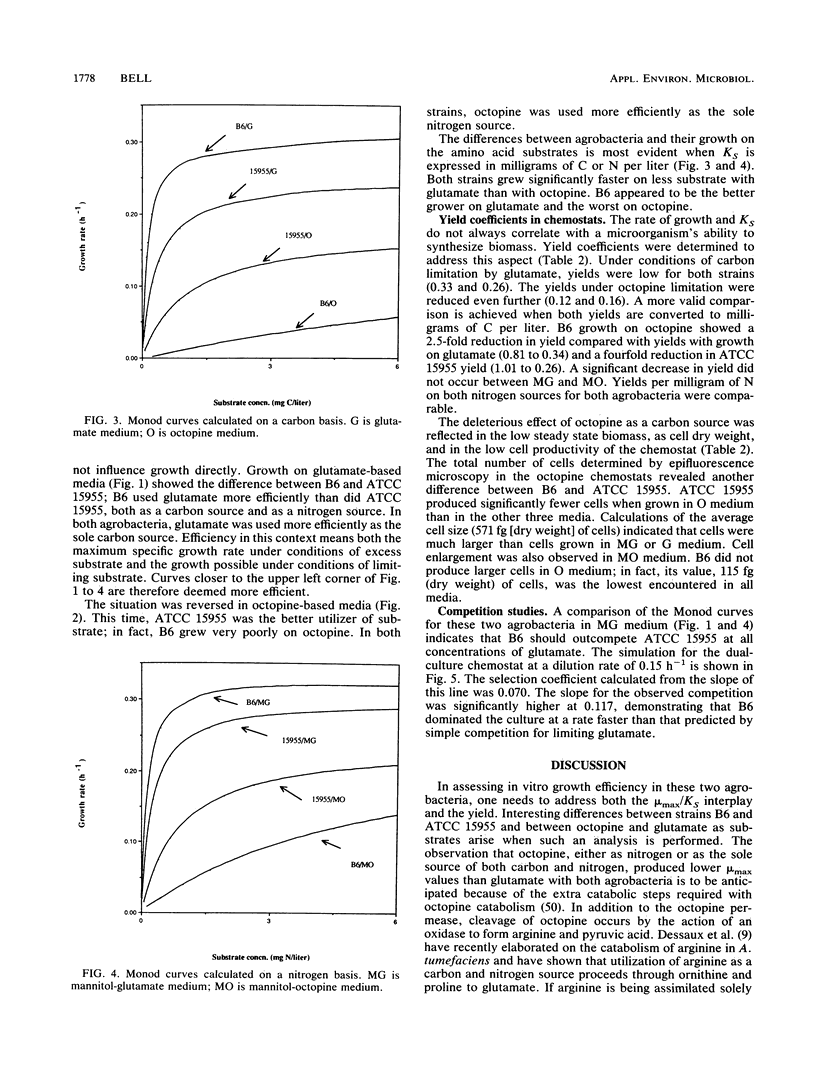
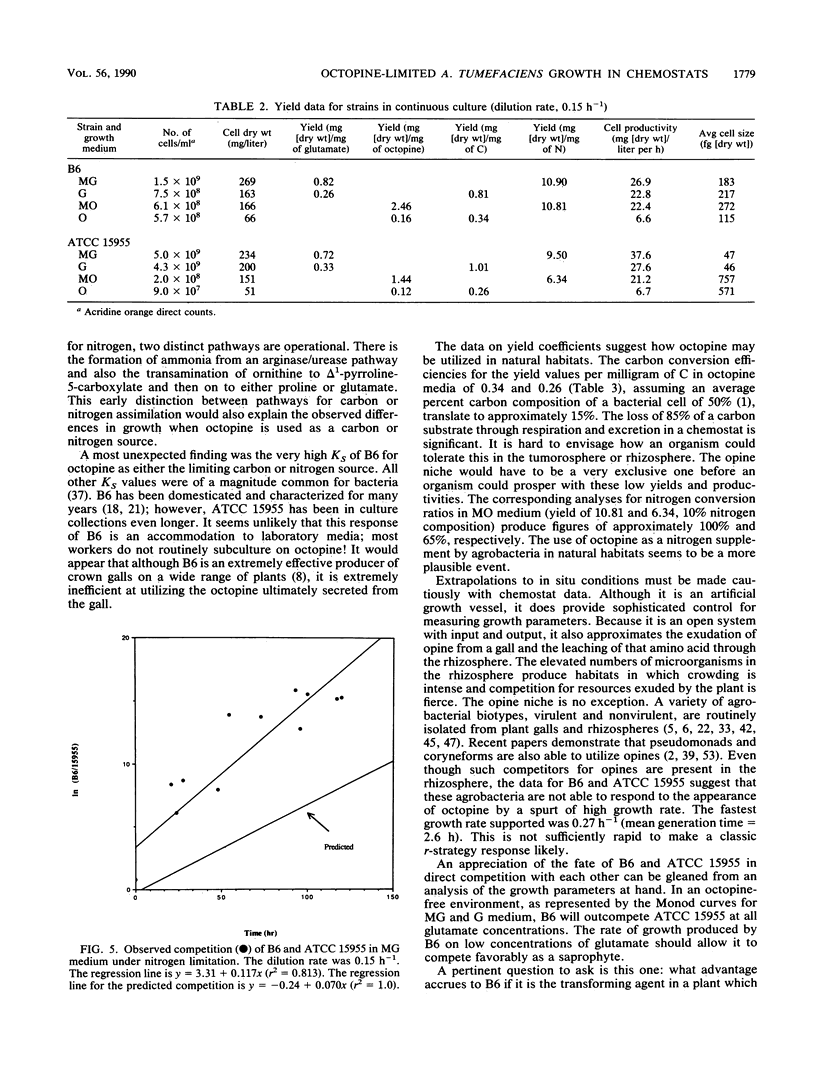
Agrobacterium tumefaciens B6 and ATCC 15955 were grown under octopine or glutamate limitation in chemostats. Examination of the maximum specific growth rate (mu max) and substrate affinity (KS) for each strain indicated that strain B6 was highly inefficient in its use of octopine as either a nitrogen or carbon source compared with strain ATCC 15955. Examination of the yield coefficients showed that in both strains octopine was used more efficiently as a nitrogen source than as a carbon source. The data permitted predictions to be made concerning the outcome of competition for a single limiting substrate. Under octopine limitation, strain ATCC 15955 should dominate; under glutamate limitation, strain B6 should dominate. The results of an observed competition with glutamate as the limiting substrate confirmed the latter prediction, although B6 did dominate at a rate faster than was predicted from simple competition theory. B6 displayed higher growth rates and substrate affinities than ATCC 15955 on all concentrations of glutamate. The yield of B6 on octopine was also considerably higher. This latter attribute could provide an ecological advantage to B6 because of the importance of yield in the fate of competitions under multisubstrate regimens. These will be the most prevalent regimens in the area around the tumor (tumorosphere) or the rhizosphere. The increased performance on glutamate could provide an advantage in an opine-free environment when B6 is growing as a saprophyte.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bomhoff G., Klapwijk P. M., Kester H. C., Schilperoort R. A., Hernalsteens J. P., Schell J. Octopine and nopaline synthesis and breakdown genetically controlled by a plasmid of Agrobacterium tumefaciens. Mol Gen Genet. 1976 May 7;145(2):177–181. doi: 10.1007/BF00269591. [DOI] [PubMed] [Google Scholar]
- Bouzar H., Moore L. W. Isolation of different agrobacterium biovars from a natural oak savanna and tallgrass prairie. Appl Environ Microbiol. 1987 Apr;53(4):717–721. doi: 10.1128/aem.53.4.717-721.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilton W. S., Chilton M. D. Mannityl opine analogs allow isolation of catabolic pathway regulatory mutants. J Bacteriol. 1984 May;158(2):650–658. doi: 10.1128/jb.158.2.650-658.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessaux Y., Petit A., Tempé J., Demarez M., Legrain C., Wiame J. M. Arginine catabolism in Agrobacterium strains: role of the Ti plasmid. J Bacteriol. 1986 Apr;166(1):44–50. doi: 10.1128/jb.166.1.44-50.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykhuizen D. E., Hartl D. L. Selection in chemostats. Microbiol Rev. 1983 Jun;47(2):150–168. doi: 10.1128/mr.47.2.150-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykhuizen D., Hartl D. L. Selective neutrality of 6PGD allozymes in E. coli and the effects of genetic background. Genetics. 1980 Dec;96(4):801–817. doi: 10.1093/genetics/96.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J. G., Kerr A., Tempé J., Petit A. Arginine catabolism: a new function of both octopine and nopaline Ti-plasmids of Agrobacterium. Mol Gen Genet. 1979 Jun 20;173(3):263–269. doi: 10.1007/BF00268636. [DOI] [PubMed] [Google Scholar]
- Garfinkel D. J., Nester E. W. Agrobacterium tumefaciens mutants affected in crown gall tumorigenesis and octopine catabolism. J Bacteriol. 1980 Nov;144(2):732–743. doi: 10.1128/jb.144.2.732-743.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyon P., Chilton M. D., Petit A., Tempé J. Agropine in "null-type" crown gall tumors: Evidence for generality of the opine concept. Proc Natl Acad Sci U S A. 1980 May;77(5):2693–2697. doi: 10.1073/pnas.77.5.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S. E., Farrand S. K. Diversity among B6 strains of Agrobacterium tumefaciens. J Bacteriol. 1980 Mar;141(3):1127–1133. doi: 10.1128/jb.141.3.1127-1133.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R., Guderian R. H., Eden F., Chilton M. D., Gordon M. P., Nester E. W. Detection and quantitation of octopine in normal plant tissue and in crown gall tumors. Proc Natl Acad Sci U S A. 1974 Feb;71(2):536–539. doi: 10.1073/pnas.71.2.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapwijk P. M., Hooykaas P. J., Kester H. C., Schilperoort R. A., RORSCH A. Isolation and characterization of Agrobacterium tumefaciens mutants affected in the utilization of octopine, octopinic acid and lysopine. J Gen Microbiol. 1976 Sep;96(1):155–163. doi: 10.1099/00221287-96-1-155. [DOI] [PubMed] [Google Scholar]
- Klapwijk P. M., Scheulderman T., Schilperoort R. A. Coordinated regulation of octopine degradation and conjugative transfer of Ti plasmids in Agrobacterium tumefaciens: evidence for a common regulatory gene and separate operons. J Bacteriol. 1978 Nov;136(2):775–785. doi: 10.1128/jb.136.2.775-785.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott J. A., Beiderbeck R., Lippincott B. B. Utilization of octopine and nopaline by Agrobacterium. J Bacteriol. 1973 Oct;116(1):378–383. doi: 10.1128/jb.116.1.378-383.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megee R. D., 3rd, Drake J. F., Fredrickson A. G., Tsuchiya H. M. Studies in intermicrobial symbiosis. Saccharomyces cerevisiae and Lactobacillus casei. Can J Microbiol. 1972 Nov;18(11):1733–1742. doi: 10.1139/m72-269. [DOI] [PubMed] [Google Scholar]
- Montoya A. L., Chilton M. D., Gordon M. P., Sciaky D., Nester E. W. Octopine and nopaline metabolism in Agrobacterium tumefaciens and crown gall tumor cells: role of plasmid genes. J Bacteriol. 1977 Jan;129(1):101–107. doi: 10.1128/jb.129.1.101-107.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya A. L., Moore L. W., Gordon M. P., Nester E. W. Multiple genes coding for octopine-degrading enzymes in Agrobacterium. J Bacteriol. 1978 Dec;136(3):909–915. doi: 10.1128/jb.136.3.909-915.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murai N., Kemp J. D. Octopine synthase mRNA isolated from sunflower crown gall callus is homologous to the Ti plasmid of Agrobacterium tumefaciens. Proc Natl Acad Sci U S A. 1982 Jan;79(1):86–90. doi: 10.1073/pnas.79.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy P. J., Heycke N., Banfalvi Z., Tate M. E., de Bruijn F., Kondorosi A., Tempé J., Schell J. Genes for the catabolism and synthesis of an opine-like compound in Rhizobium meliloti are closely linked and on the Sym plasmid. Proc Natl Acad Sci U S A. 1987 Jan;84(2):493–497. doi: 10.1073/pnas.84.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesme X., Michel M. F., Digat B. Population Heterogeneity of Agrobacterium tumefaciens in Galls of Populus L. from a Single Nursery. Appl Environ Microbiol. 1987 Apr;53(4):655–659. doi: 10.1128/aem.53.4.655-659.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong S. A., Peterson T., Neilands J. B. Agrobactin, a siderophore from Agrobacterium tumefaciens. J Biol Chem. 1979 Mar 25;254(6):1860–1865. [PubMed] [Google Scholar]
- POWELL E. O. Criteria for the growth of contaminants and mutants in continuous culture. J Gen Microbiol. 1958 Feb;18(1):259–268. doi: 10.1099/00221287-18-1-259. [DOI] [PubMed] [Google Scholar]
- Schell J., Van Montagu M., De Beuckeleer M., De Block M., Depicker A., De Wilde M., Engler G., Genetello C., Hernalsteens J. P., Holsters M. Interactions and DNA transfer between Agrobacterium tumefaciens, the Ti-plasmid and the plant host. Proc R Soc Lond B Biol Sci. 1979 Apr 11;204(1155):251–266. doi: 10.1098/rspb.1979.0026. [DOI] [PubMed] [Google Scholar]
- Taylor P. A., leB Williams P. J. Theoretical studies on the coexistence of competing species under continuous-flow conditions. Can J Microbiol. 1975 Jan;21(1):90–98. doi: 10.1139/m75-013. [DOI] [PubMed] [Google Scholar]
- Tremblay G., Gagliardo R., Chilton W. S., Dion P. Diversity among Opine-Utilizing Bacteria: Identification of Coryneform Isolates. Appl Environ Microbiol. 1987 Jul;53(7):1519–1524. doi: 10.1128/aem.53.7.1519-1524.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


