Abstract
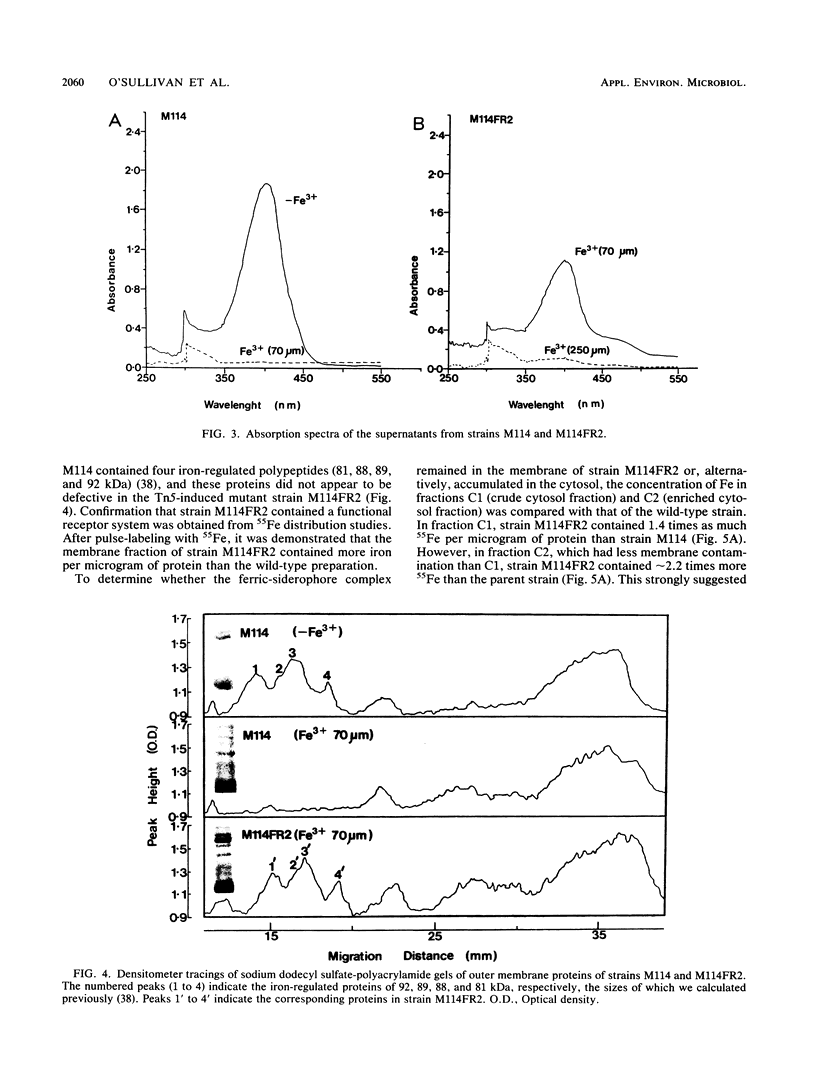
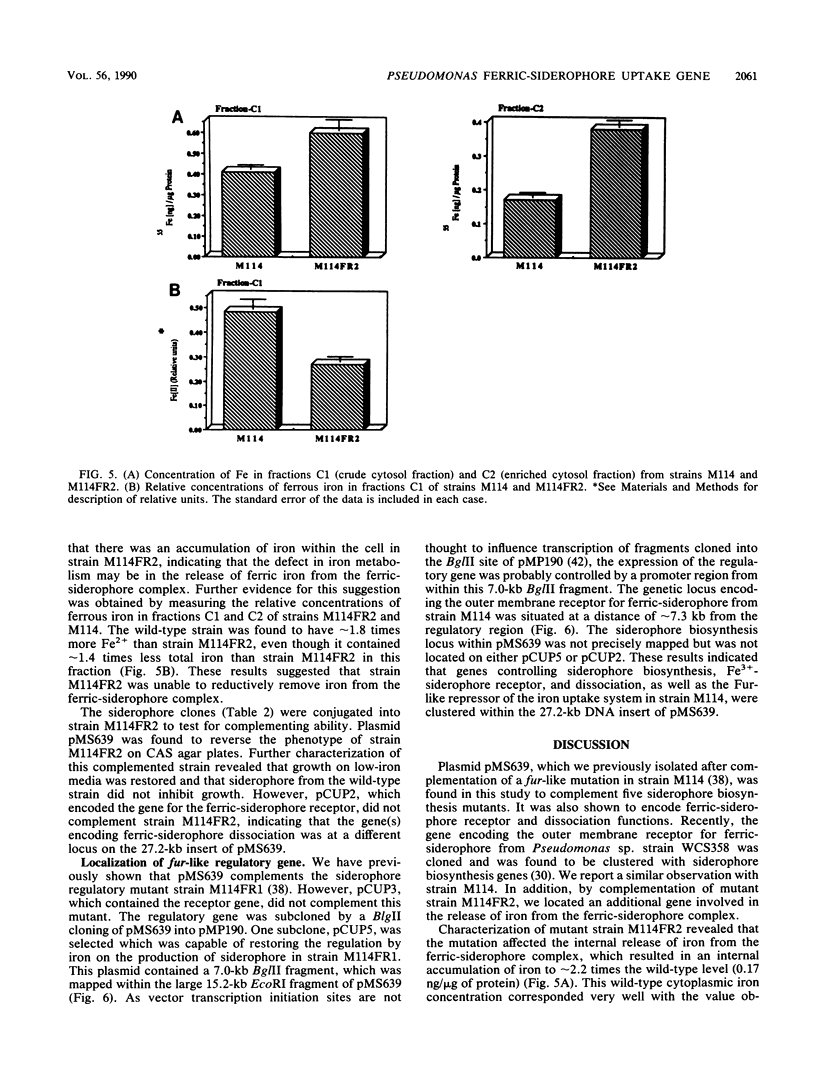
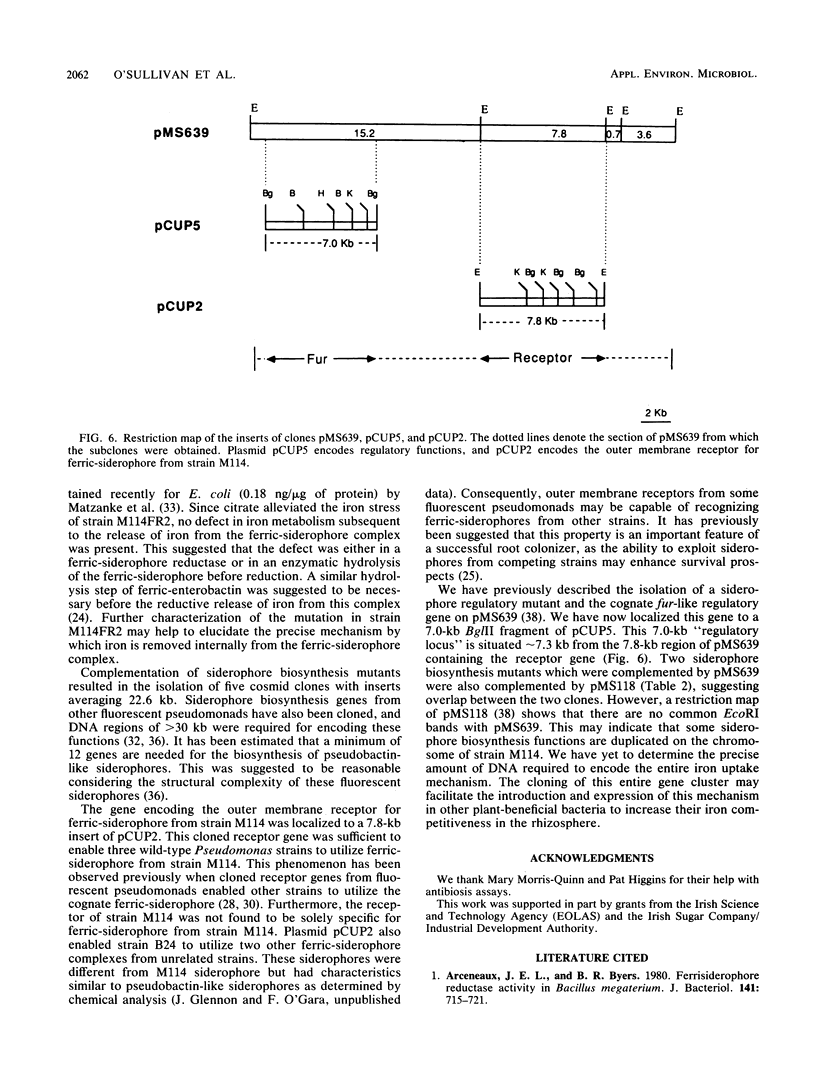
Five cosmid clones with insert sizes averaging 22.6 kilobases (kb) were isolated after complementation of 22 Tn5-induced Sid- mutants of Pseudomonas sp. strain M114. One of these plasmids (pMS639) was also shown to encode ferric-siderophore receptor and dissociation functions. The receptor gene was located on this plasmid since introduction of the plasmid into three wild-type fluorescent pseudomonads enabled them to utilize the ferric-siderophore from strain M114. The presence of an extra iron-regulated protein in the outer membrane profile of one of these strains was detected by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. A ferric-siderophore dissociation gene was attributed to pMS639 since it complemented the ferric-siderophore uptake mutation in strain M114FR2. This mutant was not defective in the outer membrane receptor for ferric-siderophore but apparently accumulated ferric-siderophore internally. Since ferric-citrate alleviated the iron stress of the mutant, there was no defect in iron metabolism subsequent to release of iron from the ferric-siderophore complex. Consequently, this mutant was defective in ferric-siderophore dissociation. A fur-like regulatory gene also present on pMS639 was subcloned to a 7.0-kb BglII insert of pCUP5 and was located approximately 7.3 kb from the receptor region. These results established that the 27.2-kb insert of pMS639 encoded at least two siderophore biosynthesis genes, ferric-siderophore receptor and dissociation genes, and a fur-like regulatory gene from the biocontrol fluorescent Pseudomonas sp. strain M114.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arceneaux J. E., Byers B. R. Ferrisiderophore reductase activity in Bacillus megaterium. J Bacteriol. 1980 Feb;141(2):715–721. doi: 10.1128/jb.141.2.715-721.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdasarian M., Lurz R., Rückert B., Franklin F. C., Bagdasarian M. M., Frey J., Timmis K. N. Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene. 1981 Dec;16(1-3):237–247. doi: 10.1016/0378-1119(81)90080-9. [DOI] [PubMed] [Google Scholar]
- Bagg A., Neilands J. B. Mapping of a mutation affecting regulation of iron uptake systems in Escherichia coli K-12. J Bacteriol. 1985 Jan;161(1):450–453. doi: 10.1128/jb.161.1.450-453.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Calderwood S. B., Mekalanos J. J. Iron regulation of Shiga-like toxin expression in Escherichia coli is mediated by the fur locus. J Bacteriol. 1987 Oct;169(10):4759–4764. doi: 10.1128/jb.169.10.4759-4764.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Chart H., Trust T. J. Acquisition of iron by Aeromonas salmonicida. J Bacteriol. 1983 Nov;156(2):758–764. doi: 10.1128/jb.156.2.758-764.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C. D. Iron reductases from Pseudomonas aeruginosa. J Bacteriol. 1980 Jan;141(1):199–204. doi: 10.1128/jb.141.1.199-204.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst J. F., Winkelmann G. Enzymatic release of iron from sideramines in fungi. NADH:sideramine oxidoreductase in Neurospora crassa. Biochim Biophys Acta. 1977 Nov 7;500(1):27–41. doi: 10.1016/0304-4165(77)90043-5. [DOI] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griggs D. W., Konisky J. Mechanism for iron-regulated transcription of the Escherichia coli cir gene: metal-dependent binding of fur protein to the promoters. J Bacteriol. 1989 Feb;171(2):1048–1054. doi: 10.1128/jb.171.2.1048-1054.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K. Cloning of the repressor protein gene of iron-regulated systems in Escherichia coli K12. Mol Gen Genet. 1984;197(2):337–341. doi: 10.1007/BF00330982. [DOI] [PubMed] [Google Scholar]
- Huyer M., Page W. J. Ferric reductase activity in Azotobacter vinelandii and its inhibition by Zn2+. J Bacteriol. 1989 Jul;171(7):4031–4037. doi: 10.1128/jb.171.7.4031-4037.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING E. O., WARD M. K., RANEY D. E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954 Aug;44(2):301–307. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laird A. J., Ribbons D. W., Woodrow G. C., Young I. G. Bacteriophage Mu-mediated gene transposition and in vitro cloning of the enterochelin gene cluster of Escherichia coli. Gene. 1980 Nov;11(3-4):347–357. doi: 10.1016/0378-1119(80)90074-8. [DOI] [PubMed] [Google Scholar]
- Langman L., Young I. G., Frost G. E., Rosenberg H., Gibson F. Enterochelin system of iron transport in Escherichia coli: mutations affecting ferric-enterochelin esterase. J Bacteriol. 1972 Dec;112(3):1142–1149. doi: 10.1128/jb.112.3.1142-1149.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge J. S., Gaines C. G., Arceneaux J. E., Byers B. R. Ferrisiderophore reductase activity in Agrobacterium tumefaciens. J Bacteriol. 1982 Feb;149(2):771–774. doi: 10.1128/jb.149.2.771-774.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magazin M. D., Moores J. C., Leong J. Cloning of the gene coding for the outer membrane receptor protein for ferric pseudobactin, a siderophore from a plant growth-promoting Pseudomonas strain. J Biol Chem. 1986 Jan 15;261(2):795–799. [PubMed] [Google Scholar]
- Marugg J. D., Nielander H. B., Horrevoets A. J., van Megen I., van Genderen I., Weisbeek P. J. Genetic organization and transcriptional analysis of a major gene cluster involved in siderophore biosynthesis in Pseudomonas putida WCS358. J Bacteriol. 1988 Apr;170(4):1812–1819. doi: 10.1128/jb.170.4.1812-1819.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marugg J. D., de Weger L. A., Nielander H. B., Oorthuizen M., Recourt K., Lugtenberg B., van der Hofstad G. A., Weisbeek P. J. Cloning and characterization of a gene encoding an outer membrane protein required for siderophore-mediated uptake of Fe3+ in Pseudomonas putida WCS358. J Bacteriol. 1989 May;171(5):2819–2826. doi: 10.1128/jb.171.5.2819-2826.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marugg J. D., van Spanje M., Hoekstra W. P., Schippers B., Weisbeek P. J. Isolation and analysis of genes involved in siderophore biosynthesis in plant-growth-stimulating Pseudomonas putida WCS358. J Bacteriol. 1985 Nov;164(2):563–570. doi: 10.1128/jb.164.2.563-570.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzanke B. F., Müller G. I., Bill E., Trautwein A. X. Iron metabolism of Escherichia coli studied by Mössbauer spectroscopy and biochemical methods. Eur J Biochem. 1989 Aug 1;183(2):371–379. doi: 10.1111/j.1432-1033.1989.tb14938.x. [DOI] [PubMed] [Google Scholar]
- Moody M. D., Dailey H. A. Ferric iron reductase of Rhodopseudomonas sphaeroides. J Bacteriol. 1985 Sep;163(3):1120–1125. doi: 10.1128/jb.163.3.1120-1125.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moores J. C., Magazin M., Ditta G. S., Leong J. Cloning of genes involved in the biosynthesis of pseudobactin, a high-affinity iron transport agent of a plant growth-promoting Pseudomonas strain. J Bacteriol. 1984 Jan;157(1):53–58. doi: 10.1128/jb.157.1.53-58.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettis G. S., McIntosh M. A. Molecular characterization of the Escherichia coli enterobactin cistron entF and coupled expression of entF and the fes gene. J Bacteriol. 1987 Sep;169(9):4154–4162. doi: 10.1128/jb.169.9.4154-4162.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwyn B., Neilands J. B. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987 Jan;160(1):47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- Tait G. H. The identification and biosynthesis of siderochromes formed by Micrococcus denitrificans. Biochem J. 1975 Jan;146(1):191–204. doi: 10.1042/bj1460191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann L., Hantke K., Braun V. Exogenous induction of the iron dicitrate transport system of Escherichia coli K-12. J Bacteriol. 1984 Jul;159(1):271–277. doi: 10.1128/jb.159.1.271-277.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]





