Abstract
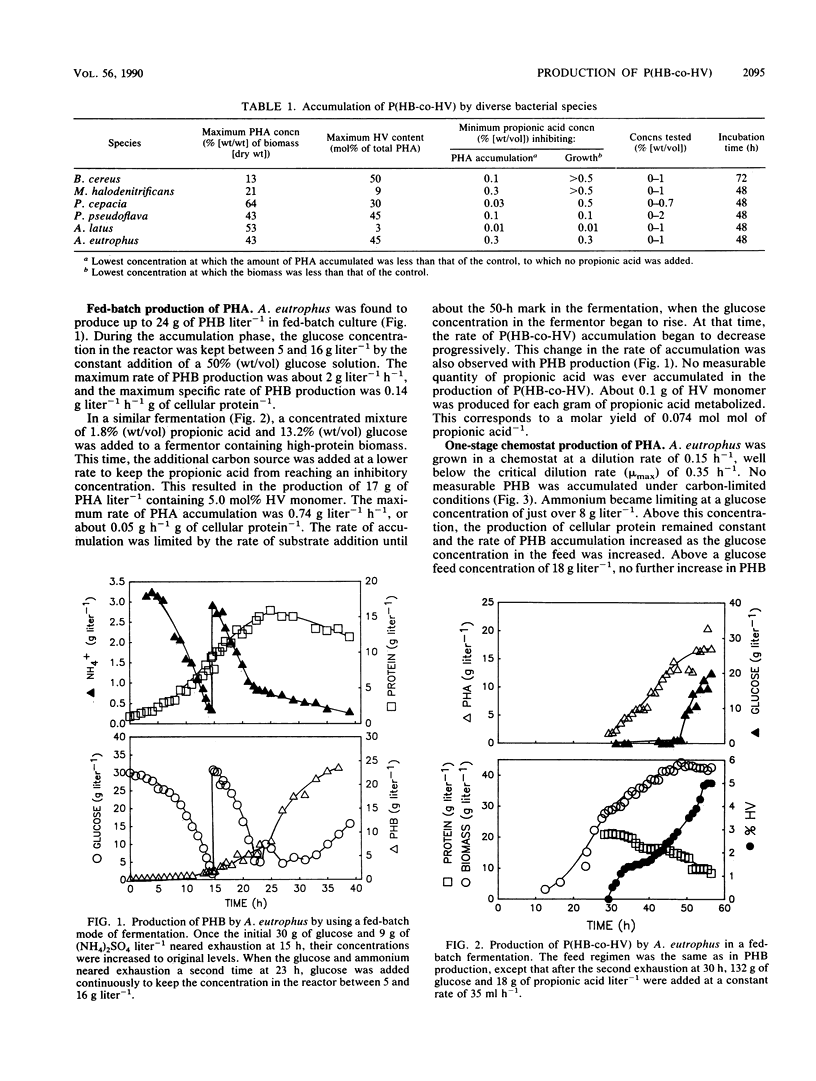
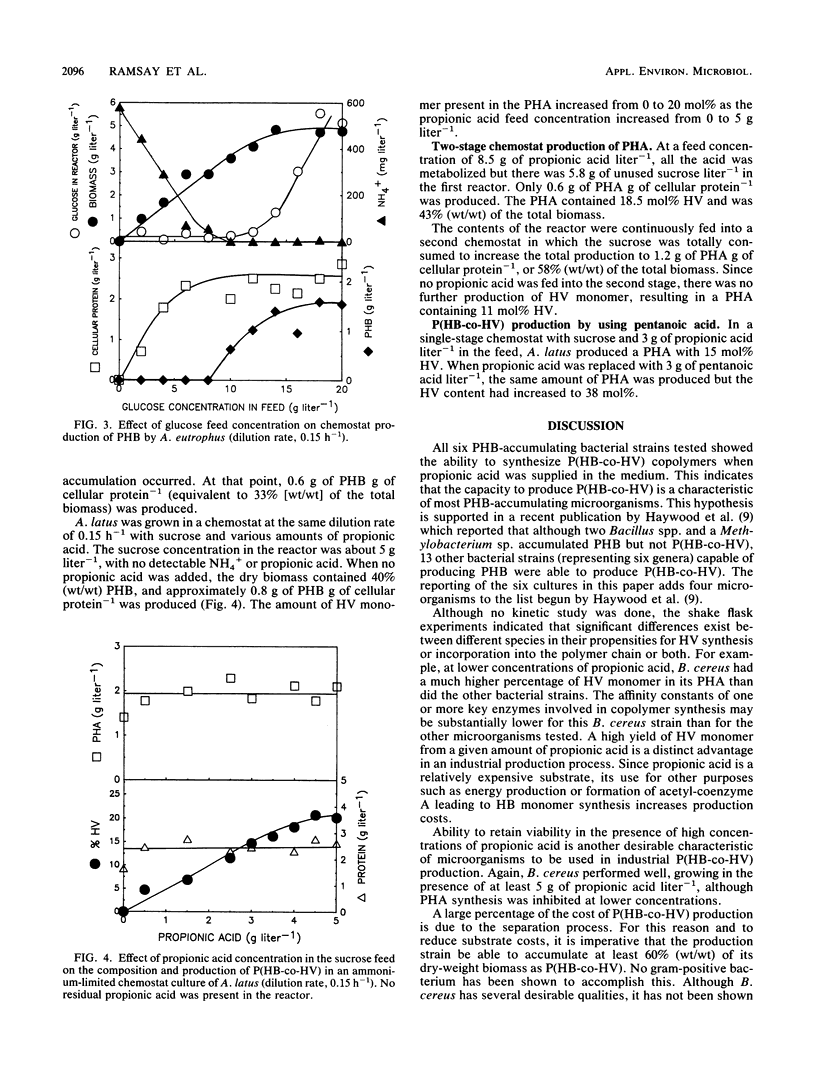
Alcaligenes latus, Alcaligenes eutrophus, Bacillus cereus, Pseudomonas pseudoflava, Pseudomonas cepacia, and Micrococcus halodenitrificans were found to accumulate poly-(beta-hydroxybutyric-co-beta-hydroxyvaleric) acid [P(HB-co-HV)] copolymer when supplied with glucose (or sucrose in the case of A. latus) and propionic acid under nitrogen-limited conditions. A fed-batch culture of A. eutrophus produced 24 g of poly-beta-hydroxybutyric acid (PHB) liter-1 under ammonium limitation conditions. When the glucose feed was replaced with glucose and propionic acid during the polymer accumulation phase, 17 g of P(HB-co-HV) liter-1 was produced. The P(HB-co-HV) contained 5.0 mol% beta-hydroxyvaleric acid (HV). Varying the carbon-to-nitrogen ratio at a dilution rate of 0.15 h-1 in a chemostat culture of A. eutrophus resulted in a maximum value of 33% (wt/wt) PHB in the biomass. In comparison, A. latus accumulated about 40% (wt/wt) PHB in chemostat culture under nitrogen-limited conditions at the same dilution rate. When propionic acid was added to the first stage of a two-stage chemostat, A. latus produced 43% (wt/wt) P(HB-co-HV) containing 18.5 mol% HV. In the second stage, the P(HB-co-HV) increased to 58% (wt/wt) with an HV content of 11 mol% without further addition of carbon substrate. The HV composition in P(HB-co-HV) was controlled by regulating the concentration of propionic acid in the feed. Poly-beta-hydroxyalkanoates containing a higher percentage of HV were produced when pentanoic acid replaced propionic acid.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dawes E. A., Senior P. J. The role and regulation of energy reserve polymers in micro-organisms. Adv Microb Physiol. 1973;10:135–266. doi: 10.1016/s0065-2911(08)60088-0. [DOI] [PubMed] [Google Scholar]
- Fay J. P., Farias R. N. The inhibitory action of fatty acids on the growth of Escherichia coli. J Gen Microbiol. 1975 Dec;91(2):233–240. doi: 10.1099/00221287-91-2-233. [DOI] [PubMed] [Google Scholar]
- Ramsay B. A., Znoj G. M., Cooper D. G. Use of a nylon manufacturing waste as an industrial fermentation substrate. Appl Environ Microbiol. 1986 Jul;52(1):152–156. doi: 10.1128/aem.52.1.152-156.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STICKLAND L. H. The determination of small quantities of bacteria by means of the biuret reaction. J Gen Microbiol. 1951 Oct;5(4):698–703. doi: 10.1099/00221287-5-4-698. [DOI] [PubMed] [Google Scholar]


