Abstract
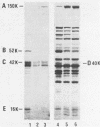
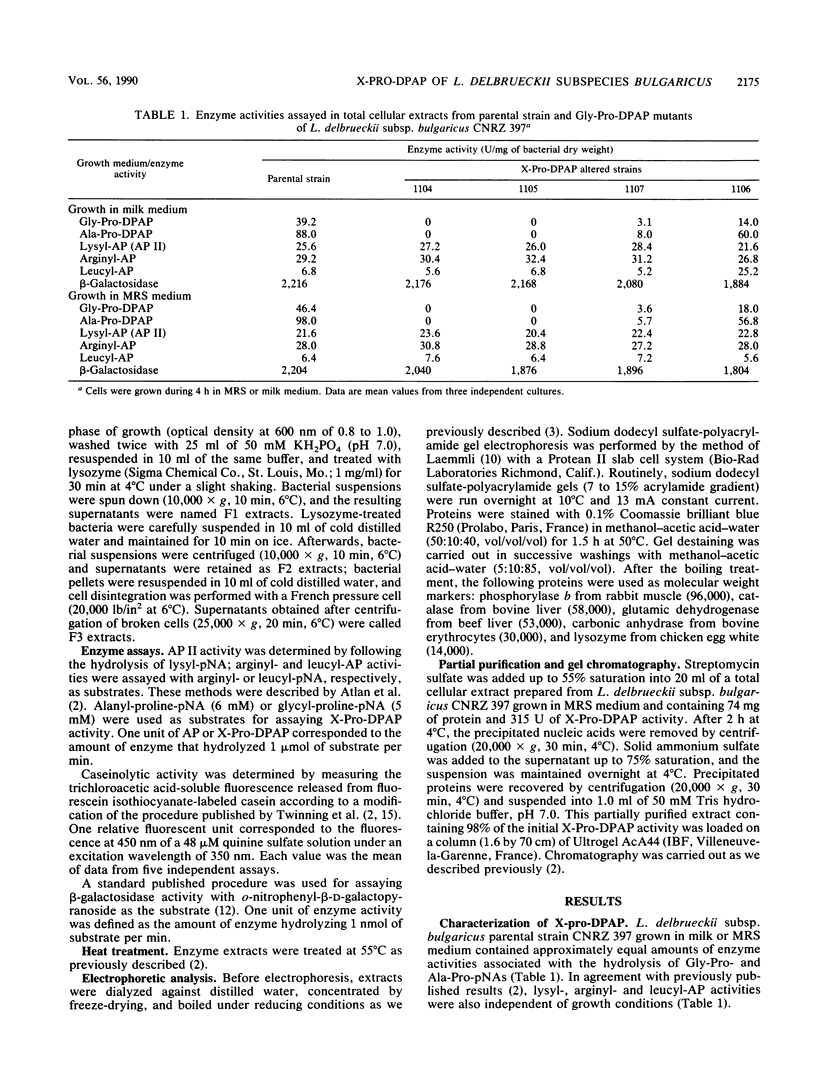
Lactobacillus delbrueckii subsp. bulgaricus CNRZ 397 is able to hydrolyze X-proline-para-nitroanilides and X-proline-β-naphthylamides (X for alanyl- or glycyl-). A single metal-independent cytoplasmic enzyme with a molecular weight estimated to be 82,000 is responsible for these activities and was named X-prolyl-dipeptidyl aminopeptidase (X-Pro-DPAP). Isolation and analysis of mutants totally deficient for X-Pro-DPAP activity showed that a total lack of this enzyme induces (i) a decrease in the growth rate; (ii) an increase in cell wall proteinase activity; (iii) the loss of three cell wall proteins with respective molecular masses of 16, 40, and 52 kilodaltons; and (iv) enhancement of a cell wall protein with a molecular mass of 150 kilodaltons. The involvement of X-Pro-DPAP in casein catabolism is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atlan D., Laloi P., Portalier R. Isolation and Characterization of Aminopeptidase-Deficient Lactobacillus bulgaricus Mutants. Appl Environ Microbiol. 1989 Jul;55(7):1717–1723. doi: 10.1128/aem.55.7.1717-1723.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CITTI J. E., SANDINE W. E., ELLIKER P. R. BETA-GALACTOSIDASE OF STREPTOCOCCUS LACTIS. J Bacteriol. 1965 Apr;89:937–942. doi: 10.1128/jb.89.4.937-942.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey M. G., Meyer J. Presence of X-prolyl-dipeptidyl-peptidase in lactic acid bacteria. J Dairy Sci. 1985 Dec;68(12):3212–3215. doi: 10.3168/jds.S0022-0302(85)81229-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Meyer J., Jordi R. Purification and characterization of X-prolyl-dipeptidyl-aminopeptidase from Lactobacillus lactis and from Streptococcus thermophilus. J Dairy Sci. 1987 Apr;70(4):738–745. doi: 10.3168/jds.S0022-0302(87)80068-1. [DOI] [PubMed] [Google Scholar]
- Schacterle G. R., Pollack R. L. A simplified method for the quantitative assay of small amounts of protein in biologic material. Anal Biochem. 1973 Feb;51(2):654–655. doi: 10.1016/0003-2697(73)90523-x. [DOI] [PubMed] [Google Scholar]
- Twining S. S. Fluorescein isothiocyanate-labeled casein assay for proteolytic enzymes. Anal Biochem. 1984 Nov 15;143(1):30–34. doi: 10.1016/0003-2697(84)90553-0. [DOI] [PubMed] [Google Scholar]