Abstract
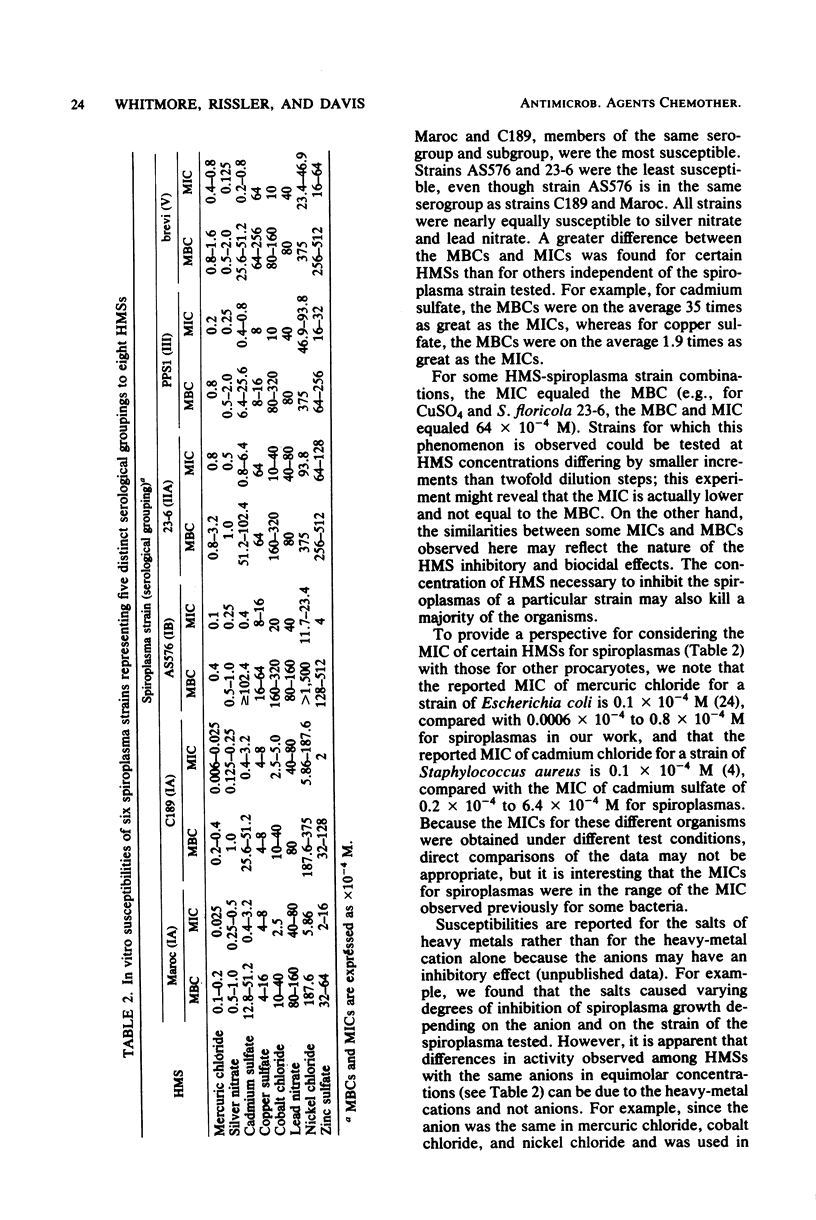
The susceptibility of six spiroplasma strains to heavy-metal salt was characterized in terms of minimal inhibitory concentrations and minimal biocidal concentrations in broth tube dilution tests. The strains were most susceptible to mercuric chloride and silver nitrate; less susceptible to copper sulfate, cobalt chloride, lead nitrate, and cadmium sulfate; and least susceptible to nickel chloride and zinc sulfate. Spiroplasma citri strains Maroc R8A2 and C189 were the most susceptible to five of eight heavy-metal salts, and honeybee spiroplasma strain AS576 and Spiroplasma floricola strain 23-6 were generally the least susceptible. The difference between the minimal biocidal concentrations and the minimal inhibitory concentrations was greater for certain heavy-metal salts than for others.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chopra I. Mechanism of plasmic-mediated resistance to cadmium in Staphylococcus aureus. Antimicrob Agents Chemother. 1975 Jan;7(1):8–14. doi: 10.1128/aac.7.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. E., Lee I. M., Basciano L. K. Spiroplasmas: serological grouping of strains associated with plants and insects. Can J Microbiol. 1979 Aug;25(8):861–866. doi: 10.1139/m79-128. [DOI] [PubMed] [Google Scholar]
- Davis R. E., Lee I. M. Comparative properties of spiroplasmas and emerging taxonomic concepts: a proposal. Rev Infect Dis. 1982 May-Jun;4 (Suppl):S122–S128. doi: 10.1093/clinids/4.supplement_1.s122. [DOI] [PubMed] [Google Scholar]
- Davis R. E. Spiroplasma associated with flowers of the tulip tree (Liriodendron tulipifera L.). Can J Microbiol. 1978 Aug;24(8):954–959. doi: 10.1139/m78-158. [DOI] [PubMed] [Google Scholar]
- Davis R. E., Worley J. F., Whitcomb R. F., Ishijima T., Steere R. L. Helical filaments produced by a Mycoplasma-like organism associated with corn stunt disease. Science. 1972 May 5;176(4034):521–523. doi: 10.1126/science.176.4034.521. [DOI] [PubMed] [Google Scholar]
- Lee I. M., Davis R. E. DNA homology among diverse spiroplasma strains representing several serological groups. Can J Microbiol. 1980 Nov;26(11):1356–1363. doi: 10.1139/m80-224. [DOI] [PubMed] [Google Scholar]
- Ranhand J. M., Mitchell W. O., Popkin T. J., Cole R. M. Covalently closed circular deoxyribonucleic acids in spiroplasmas. J Bacteriol. 1980 Sep;143(3):1194–1199. doi: 10.1128/jb.143.3.1194-1199.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitcomb R. F. The genus Spiroplasma. Annu Rev Microbiol. 1980;34:677–709. doi: 10.1146/annurev.mi.34.100180.003333. [DOI] [PubMed] [Google Scholar]
- Whitcomb R. F., Williamson D. L. Helical wall-free prokaryotes in insects: multiplication and pathogenicity. Ann N Y Acad Sci. 1975;266:260–275. doi: 10.1111/j.1749-6632.1975.tb35109.x. [DOI] [PubMed] [Google Scholar]


