Abstract
Los1p/exportin-t (XPOT) mediates the nuclear export of tRNAs in yeast and mammals. The requirements for this transport pathway are unclear, however, because los1 mutations do not affect yeast growth, and the phenotype of XPOT mutations in mammals is unknown. Here, we show that PAUSED (PSD) is the Arabidopsis ortholog of LOS1/XPOT and is capable of rescuing the tRNA export defect of los1 in Brewer's yeast (Saccharomyces cerevisiae), suggesting that its function has been conserved. Putative null alleles of PSD disrupt the initiation of the shoot apical meristem and delay leaf initiation after germination, the emergence of the radicle and lateral roots, and the transition to flowering. Plants doubly mutant for psd and hasty, the Arabidopsis ortholog of exportin 5, are viable but have a more severe phenotype than either single mutant. These results suggest that PSD plays a role in tRNA export in Arabidopsis, but that at least one—and perhaps several—additional tRNA export pathways also exist. The PSD transcript is broadly expressed during development and is alternatively spliced in the 3′-untranslated region. No temporal or spatial difference in the abundance of different splice forms was observed. We propose that the mutant phenotype of psd reflects defects in developmental events and cell/tissue types that require elevated levels of protein synthesis and are therefore acutely sensitive to a reduction in tRNA export.
Nucleocytoplasmic transport is mediated by a family of structurally related proteins known as karyopherins. These proteins, also called importins and exportins, bind a variety of protein and RNA cargoes and guide their movement through the nuclear pore. Many fundamental cellular processes, such as the nuclear import of ribosomal proteins and the export of tRNA molecules, require importin or exportin activity (for review, see Nakielny and Dreyfuss, 1999). Karyopherins also mediate nucleocytoplasmic transport in response to environmental or developmental cues. For example, in Brewer's yeast (Saccharomyces cerevisiae), the karyopherins Pse1p and Msn5p regulate the nuclear import and export of the transcription factor Pho4p in response to phosphate levels (Kaffman et al., 1998a, 1998b), and in fruitfly (Drosophila melanogaster), the importin 7 ortholog, DIM-7, and the importin-β ortholog, KETEL, are required to transport the Ser kinase d-ERK into the nucleus in response to EGFR signaling (Lorenzen et al., 2001).
The karyopherin family has been best characterized in Brewer's yeast, where it consists of 14 members. The loss-of-function phenotype has been determined for all of the members of this family, and 12 have a known transport direction and at least one identified cargo (Costanzo et al., 2001). A picture of karyopherin function in multicellular eukaryotes has been slower to emerge. Although biochemical characterization of human and Xenopus sp. karyopherins has contributed to our understanding of their regulation and molecular interactions, little is known about their role in the context of a developing organism. No karyopherin mutations have been reported in Caenorhabditis elegans, and only three karyopherin mutations have been characterized in fruitfly (Lippai et al., 2000; Stein et al., 2001). Unlike the yeast mutations, which are generally lethal or have no effect on growth, mutations in the fruitfly orthologs of CRM1/XPO1 (embargoed), IMP7 (moleskin), and KAP95/IMPβ (ketel) all cause specific developmental defects (Collier et al., 2000; Lippai et al., 2000; Tirian et al., 2000). This may suggest a more complex requirement for these molecules in multicellular organisms, an idea supported by the observation that expression of ketel is temporally and spatially regulated (Lippai et al., 2000).
The Arabidopsis genome contains approximately 17 predicted karyopherins (Bollman et al., 2003), but little is known about their functions or relationships to karyopherins in other organisms. To date, only three Arabidopsis karyopherins have been described. AtXPO1 and AtCAS have been biochemically characterized, but it is not known how mutations in these genes affect plant growth or development (Haasen et al., 1999; Haasen and Merkle, 2002). The Arabidopsis HASTY (HST) gene is the ortholog of the human gene exportin 5 (XPO5) and the Brewer's yeast bidirectional transporter MSN5. Like msn5 mutants, hst mutants are viable but have defects in many different processes (Bollman et al., 2003).
In this paper, we describe the mutant phenotype of PAUSED (PSD), a third member of the Arabidopsis karyopherin family. PSD is a widely expressed gene that encodes a protein with homology to the tRNA export receptors Los1p in Brewer's yeast and exportin-t (XPOT) in humans. We found that PSD partially rescues the tRNA export defect of los1, supporting the conclusion that PSD can function as an export receptor for tRNAs. Mutant alleles of PSD (including several that are likely to be functionally null) are viable, but have defects in a variety of developmental processes. Null mutations of LOS1 are also viable (Hurt et al., 1987), suggesting that there is more than one export receptor for tRNAs in both yeast and Arabidopsis. XPO5-related proteins are potential candidates for this second export receptor because XPO5 is capable of binding tRNAs and promoting their nuclear export under some conditions (Bohnsack et al., 2002; Calado et al., 2002; Gwizdek et al., 2002). Although plants mutant for both psd and hst, the Arabidopsis ortholog of XPO5 (Bollman et al., 2003), had a more severe phenotype than either single mutant, these plants were viable. This result suggests that PSD and HST either have a different function than their homologs in other species, or that the nuclear export of tRNAs in Arabidopsis does not depend entirely on these two proteins.
RESULTS
psd Disrupts the Onset of Shoot Growth
PSD was identified in a screen for mutations that affect meristem initiation during embryogenesis. An initial characterization of the psd-1 mutant phenotype demonstrated that psd transiently disrupts the organization of the shoot apical meristem (SAM) and delays leaf production, but does not have a significant effect on the timing of the transition to the adult phase of vegetative development (Telfer et al., 1997). As a result, mutant plants produce their first adult leaves at inappropriately basal positions; e.g. node 1 or 2 instead of node 3 or 4. Three additional alleles of psd were subsequently identified in screens for mutations that exhibit adult characteristics on the first two leaves. These alleles are phenotypically indistinguishable from each other, and from psd-1. On the basis of molecular data (see below), we selected the psd-6 and psd-13 alleles for further analysis.
psd Delays the Appearance of Leaf Primordia
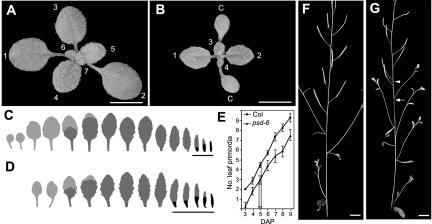
In a Columbia (Col) background, psd mutations delay the appearance of the first two leaves by approximately 2 d (Fig. 1, A–D; Table I). To determine whether the delay in leaf emergence resulted from a failure in leaf initiation or a decreased rate of leaf expansion, we examined leaf initiation in psd-6 plants carrying a LFY:GUS transgene as a marker for leaf primordia (Blazquez et al., 1997). On average, LFY: GUS positive leaf primordia were visible in Col plants 3 d after planting (DAP) but were not apparent in psd-6 until 4 DAP (Fig. 1E). Unlike wild-type plants, which initiate the first two leaves almost simultaneously, psd-6 plants tend to produce leaves 1 and 2 on consecutive days, a pattern typical of later leaves in wild-type plants. After this initial delay, psd-6 mutants produced leaf primordia at the same rate as wild-type plants. They also appeared to undergo leaf expansion at a normal rate: Leaves were visible approximately 1.3 d after initiation in both Col and psd-6 (data not shown).
Figure 1.
The psd mutation delays leaf production and disrupts inflorescence morphology. Photos of a wild-type Col plant (A) and a psd-6 mutant plant (B) 14 DAP show the reduction in leaf number caused by the delay in leaf initiation. Cotyledons (c) and leaf numbers are indicated. The resulting change in leaf shape and identity can be seen in the outlines of leaves from wild-type (C) and psd-6 (D) plants. Leaves lacking abaxial trichomes are shown in light gray, leaves with abaxial trichomes in dark gray, and leaves lacking adaxial trichomes in black. Bracts are underlined. E, The rate of initiation of LFY:GUS positive leaf primordia in Col and psd-6 shows the initial delay in the psd-6 mutant. Both wild-type and psd-6 plants initiate their first adult leaf primordium (leaf 4.9 in Col and 2.7 in psd-6) at 5 DAP (arrows). In the inflorescence of wild-type plants (F), secondary inflorescences are subtended by bracts, and siliques are distributed in an even pattern. In psd-6 (G) some secondary inflorescences lack bracts (arrow) and the phyllotaxy is disrupted (arrowhead). Bars = 5 cm for A and B and 1 cm for F and G.
Table I.
Effects of psd on Arabidopsis development
All values are mean ± se.
| Col | psd-13 | psd-6 | psd-6; [PSD+] | n | |
|---|---|---|---|---|---|
| Leaves without abaxial trichomes | 4.2±0.2 | 3.0±0.2 | 2.3±0.2 | 4.1±0.2 | 29 |
| Leaves with abaxial trichomes | 6.6±0.3 | 5.5±0.2 | 5.4±0.2 | 6.1±0.2 | 29 |
| No. of bracts | 3.3±0.2 | 4.8±0.2 | 4.7±0.2 | 2.6±0.2 | 29 |
| Days of flowering | 22.6±0.3 | 23.5±0.2 | 23.8±0.2 | 20.4±0.3 | 28 |
| Seeds per silique | 57.4±1.3 | 7.7±1.5 | 11.7±1.6 | 57.7±3.6 | 10 |
| Root length (mm), 3 d | 1.7±0.1 | 0.6±0.1 | 0.6±0.1 | 1.8±0.1 | 20 |
| Root length (mm), 14 d | 19.2±1 | 19.1±0.7 | 18.6±1.1 | 22.2±1.1 | 30 |
| No. of lateral roots, 14 d | 2.4±0.3 | 1.0±0.3 | 0.7±0.1 | 1.5±0.3 | 30 |
On average, the psd-6 plants produced their first abaxial trichomes on leaf 2.7, whereas wild-type plants first produced abaxial trichomes on leaf 4.9 (Fig. 1E). Despite this basipetal shift in the position of leaves with adult characteristics, both mutant and wild-type plants produced their first adult leaf primordium 5 DAP (Fig. 1E) and produced two transition leaves before producing leaves with a full complement of abaxial trichomes (Fig. 1, C and D). This suggests that the shift in leaf identity in psd is due to the delay in leaf production, not an alteration in the timing of the juvenile to adult transition. This observation is consistent with previous results obtained with psd-1 in a Landsberg erecta (Ler) background (Telfer et al., 1997).
psd Mutant Embryos Have an Abnormal SAM
The delay in leaf production in psd mutants is associated with disruptions in the anatomy of the SAM (Telfer et al., 1997). Sections of 3-d-old psd-6 seedlings reveal a region of dead cells in the outer layers of the central region and an expansion of the underlying cells. Cells in the peripheral region of the meristem, which gives rise to leaf primordia, appear to be unaffected (Fig. 2, E and F). The disruption of the central region of the meristem is also reflected in a bifurcation of the shoot axis, which is observed in approximately 10% of psd mutant plants. These plants produce two mirror image rosettes, each of which forms a primary inflorescence. We believe that this phenomenon is a consequence of the death of cells in the central region of the SAM because surgical ablation of these cells has a similar effect (Steeves and Sussex, 1989). In the majority of mutant plants, the meristem gradually regenerates, although the organization of the cell layers may remain aberrant as long as 8 DAP (Telfer et al., 1997).
Figure 2.
PSD is required in the SAM, but not the RAM. Confocal images of mature seeds stained with Hoechst show that the SAM of mature seeds (A) is greatly reduced in psd-6 embryos (B). At 3 DAP, wild-type seedlings (C) have a well-ordered SAM with visible leaf primordia, whereas in psd-6 (D), the central region of the SAM is disrupted and leaf primordia are reduced in size. At 3 DAP, a section of a wild-type seedlings (E) shows distinct cell layers in the SAM that are missing in psd-6 seedlings of the same age. This defect appears more severe in the central zone of the SAM, because intact cells in the peripheral zone have begun to initiate leaf primordia. Compared with wild type (G), the roots of psd-6 mutant seedlings (H) are slightly disordered and have an increased amount of starch at 3 DAP, although all cell layers appear to be present. The thickening of the psd-6 mutant root appears to be caused by an enlargement of cells in the cortical layer. Bars = 20 μm.
To determine when these defects in the SAM arise, we used confocal microscopy to examine the morphology of the SAM in mature embryos and young seedlings. The SAM of mature psd-6 mutant seeds is greatly reduced compared with wild type and shows no evidence of developing leaf primordia (Fig. 2, A and B). At 1 DAP, we observed a similar reduction in the size of the psd-6 SAM and evidence of the loss of nuclei in the central region (data not shown). By 3 DAP, the central region of the SAM was still disrupted, but peripheral regions were more organized, and leaf primordia were often evident (Fig. 2, C and D). Similar phenotypes were observed in the embryos and young seedlings of psd-13 mutant plants (data not shown). The effect of psd on the SAM of mature embryos indicates that the psd phenotype arises before germination, and the loss of leaf primordia is not specific to the juvenile phase of development. This conclusion is supported by the fact that psd can suppress the leaf-to-cotyledon transformation that occurs during the embryogenesis of amp1 mutant plants (Conway and Poethig, 1997).
psd Affects the Vegetative to Reproductive Transition
In addition to delaying the initiation of the SAM, psd also delays the transition from vegetative to reproductive development. Although mutant plants have fewer juvenile and adult leaves than normal, they actually have an increased number of leaves in the inflorescence (bracts; Fig. 1, C and D; Table I). Furthermore, the first bract of mutant plants sometimes resembles an adult vegetative leaf in having a very short internode, a slightly broader shape, and a residual petiole (Fig. 1, C and D). Whereas wild-type plants begin to lose adaxial trichomes at the base of the first bract and completely lack adaxial trichomes by the third bract, psd plants lose adaxial trichomes more gradually, and often still have adaxial trichomes on the fifth or sixth bract. Finally, psd-6 plants express AP1:GUS—a marker for floral induction (Hempel et al., 1997)—approximately 1 d later than normal (data not shown) and also have a 1-d delay in the appearance of the first open flower (Table I).
psd mutations also affect the morphogenesis of the inflorescence: Phyllotaxy is disrupted in about 25% of plants, and roughly 10% of secondary inflorescences lack a subtending bract (Fig. 1, F and G). In some cases, a very small piece of leaf tissue is present beneath the secondary inflorescence, suggesting that bract production was aborted. psd also reduces fertility, with psd plants containing about 85% fewer seeds per silique than wild-type plants (Table I).
psd Delays Root Out-Growth
psd seeds germinate at the same frequency as wild type, but exhibit a delay in the emergence of the radicle from the seed coat, and initially produce a short, thick root (Table I). Mutant seedlings also have fewer lateral roots than normal seedlings at 14 DAP. psd does not appear to have a significant effect on the overall growth of the root, however, because there was no difference in primary root length between psd and wild-type plants at 14 DAP (Table I).
To determine whether this delay in root emergence is associated with a defect in meristem organization, we examined the structure of psd-6 roots 3 DAP. In contrast to the SAM, there was no sign of cell death in the root apical meristem (RAM) of psd-6 plants: All of the cell layers were present, although cells appeared larger than wild type (Fig. 2, G and H), and there was significantly more starch in the cortex of mutant roots. It is unclear whether this phenotype reflects an effect of psd on root morphogenesis or whether the initial delay in root growth results in mutant roots being developmentally less advanced than wild-type roots. In either case, this result suggests that PSD is not required by the RAM in the same way that it is required in the SAM.
PSD Is Similar to XPOT
PSD maps near the marker nga111 at the bottom of chromosome 1. Additional markers from this region were generated using Ler sequence information provided by Cereon; these allowed us to delimit PSD to a region spanned by the bacterial artificial chromosome (BACs) T16D11 and T10N11. Southern analysis using T16D11 as a probe revealed an inversion in the fast neutron allele, psd-6. A 2-kb HindIII fragment disrupted by this inversion was cloned and sequenced and was found to contain a portion of a predicted open reading frame. To confirm that this breakpoint was responsible for the psd phenotype, a genomic fragment containing the predicted open reading frame plus 2.8 kb of upstream sequence, and 300 bp of downstream sequence was introduced into psd-6 and was found to restore wild-type growth and fertility (Table I).
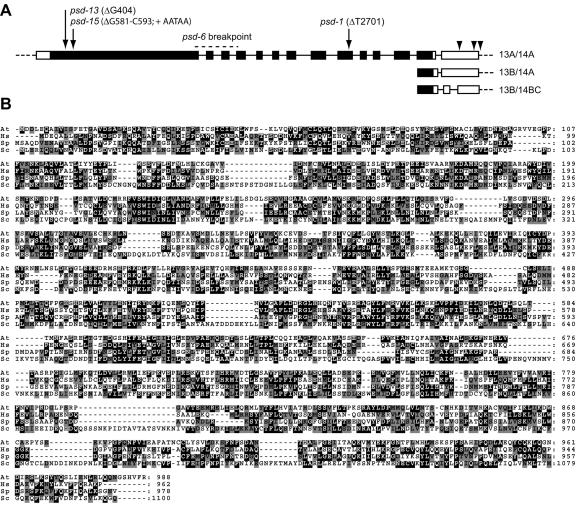
The 2-kb HindIII fragment was used to probe a seedling cDNA library, from which three partial clones were obtained, and the 5′ and 3′ ends of the transcript were isolated by RACE. The PSD transcript totals 3.6 kb and contains 14 exons; the last two exons are alternatively spliced, giving rise to multiple 3′-untranslated regions (UTRs; GenBank BK001280; Fig. 3A). The PSD cDNA encodes a predicted protein of 988 amino acids, which is 27% identical to the human tRNA nuclear export factor, XPOT (Fig. 3B). Like both yeast and humans, Arabidopsis appears to have a single XPOT gene, because there are no other genes in Arabidopsis that are more similar to LOS1/XPOT than they are to another member of the karyopherin/importin-β gene family (Bollman et al., 2003).
Figure 3.
PSD has homology to Los1p/XPOT. A, The structure of the PSD transcript is shown, with the coding region in black and the UTRs in white. The three alternate spliced forms of exons 13 and 14 are diagramed, and polyadenylation sites are shown with arrowheads. The position and nature of the four psd mutations are indicated with arrows. B, A comparison of the Arabidopsis PSD (At), human XPOT (Hs), and fission yeast (Schizosaccharomyces pombe (Sp) and Brewer's yeast (Sc) Los1p proteins. Identical amino acids boxed in black; conservative amino acid changes are shaded gray.
The DNA sequence of the other existing psd alleles revealed that all contain mutations within the same open reading frame (Fig. 3A). The EMS-induced allele psd-1 has a deletion of T2701, resulting in a premature stop codon in exon 10. One fast-neutron allele, psd-13, has a deletion of G404 and the other, psd-15, has a substitution of the sequence AATAA for the 13 bases beginning with G581. Both psd-13 and psd-15 cause premature stop codons in exon 1. We observed no difference in the phenotype of the various alleles, and given the placement of the psd-13 and psd-15 mutations, we believe that the defects described above represent the null phenotype of XPOT in Arabidopsis.
PSD Is Expressed throughout Development
A single PSD transcript of approximately 4 kb was observed on northern blots of poly(A) RNA; this transcript was reduced in both size and abundance by the psd-6 inversion (Fig. 4A). Low levels of the PSD transcript were observed in roots, vegetative leaves, and floral buds (Fig. 4B). In addition, semiquantitative RT-PCR revealed that PSD is expressed at similar levels in the shoot apex of plants grown for 8, 15, or 22 d under short-day conditions (Fig. 4C). The widespread expression of the PSD gene is not surprising, given the pleiotropic mutant phenotype and the fundamental nature of its predicted role in tRNA export.
Figure 4.
PSD is expressed throughout development. A, Northern blot showing the PSD transcript from wild-type and psd-6 mutant plants. A single transcript is detected in wild type and is reduced in size and expression level by the psd-6 inversion. B, Northern blot showing the presence of PSD transcript in the roots (Rt), rosette leaves (Lf), and floral buds (Fl) of mature plants. C, Southern blot of DdeI-digested reverse transcriptase (RT)-PCR products showing the presence of the three alternatively spliced forms of the PSD 3′-UTR in 8-, 15-, and 22-d shoot apices, and in roots, rosette leaves, and floral buds. If present, the 13A/14B form would migrate between the 13B/14A and 13B/14B forms.
The sequence of several cDNAs and 3′-RACE products revealed that the 3′-UTR of PSD is alternatively spliced (Fig. 3A). Exon 13A is 9 bp shorter than exon 13B at the 3′ end. Exon 14 is also alternatively spliced. Exon 14A extends uninterrupted to the polyadenylation site, whereas 14B1 and 14B2 are created by the presence of an additional intron that removes 88 bp from G3196 to G3283. We observed no correlation between the splicing pattern and the site of polyadenylation. Using RT-PCR, we examined the expression of the 3′-splicing variants, and found that 13A/14A, 13B/14A, and 13B/14B are present in the shoots of juvenile, adult, and reproductive plants, and in the roots, vegetative leaves, and floral buds of mature plants (Fig. 4C). The 13B/14A form appears to be the predominant form in all tissues, and we saw no evidence of the expression of the 13A/14B combination in the 3′-RACE products, cDNAs, or RT-PCR products. Together, the northern and RT-PCR data suggest that neither PSD expression nor the splicing of the 3′-UTR is developmentally regulated. However, we cannot exclude the possibility that PSD is differentially spliced in some regions of the tissues that were assayed by RT-PCR.
PSD Partially Complements the Brewer's Yeast los1 Phenotype
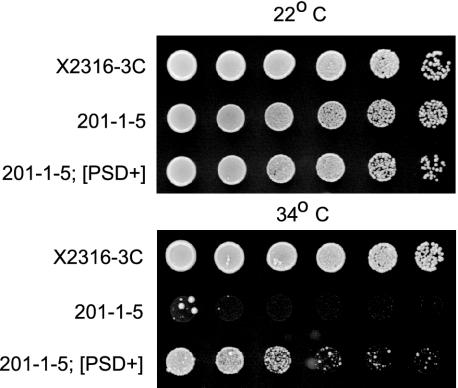
PSD is most closely related to the human XPOT gene and its Brewer's yeast ortholog LOS1. los1 mutants are viable but lack suppressor-tRNA activity due to defects in tRNA export (Hopper et al., 1980). To determine whether PSD can rescue the los1 mutant phenotype, we used the strain 201-1-5, which carries the temperature-sensitive point mutation los1-1, along with the ochre mutation ade2-1° and the ochre-suppressing tRNA gene SUP4° (Hopper et al., 1980). At nonpermissive temperatures, the los1-1 mutation blocks the export of the SUP4° tRNA, resulting in an ade2-1° phenotype of slow-growth and red pigmentation on media lacking adenine. When we introduced a construct containing the PSD open reading frame under control of the yeast alcohol dehydrogenase (ADH) promoter, we found that expression of the PSD gene partially rescued the slow-growth phenotype (Fig. 5), suggesting that the PSD protein is capable of facilitating tRNA export in a manner similar to that of its ortholog Los1p.
Figure 5.
PSD complements the Brewer's yeast los1 mutant phenotype. Serial dilutions of parent strain X2316-3C, the los1-1 temperature-sensitive mutant strain 201-1-5, or 201-1-5 containing the ADH:PSD construct were grown at permissive (22°C) or restrictive (34°C) temperature. The ADH:PSD construct is able to partially suppress the slow growth phenotype of 201-1-5 on SDC (-ade) plates.
psd;hst Double Mutants Are Viable
Like los1, psd mutants are viable, suggesting that Arabidopsis has alternate mechanisms for tRNA export. Recently, the karyopherin XPO5 has also been shown to bind tRNA (Bohnsack et al., 2002; Calado et al., 2002). Although XPO5 does not appear to increase tRNA export under normal conditions (Gwizdek et al., 2002), it does stimulate tRNA export when the XPOT pathway is impeded by saturation with exogenous tRNA (Calado et al., 2002). These observations lead us to test whether the Arabidopsis XPO5 ortholog HST (Bollman et al., 2003) provides the compensatory tRNA export function that allows the survival of psd mutant plants. For this purpose, we determined the phenotype of plants homozygous for mutant alleles of both psd and hst. These alleles truncate the predicted proteins and are likely to be functionally null because they have a phenotype that is indistinguishable from other severe alleles (Bollman et al., 2003).
Plants doubly mutant for psd-1 and hst-1 show a combination of the psd and hst mutant phenotypes in that they had the accelerated trichome production and upwardly curled leaves typical of hst but lacked their first two leaves due to the psd-related delay in leaf initiation (Fig. 6). The plants also displayed a further decrease in size compared with the single-mutant parents. This same phenotype was observed for plants doubly mutant for psd-13 and hst-6, alleles that introduce stop codons near the 5′ end of these genes (data not shown). The fact that double mutant plants are viable demonstrates that HST is not required for the survival of psd mutant plants. This could indicate that HST is not involved in tRNA transport or that it is not the only protein that can mediate an alternative tRNA export pathway in Arabidopsis.
Figure 6.
The psd-1; hst-1 double mutant plant shows a combination of the psd and hst phenotypes and is greatly reduced in size.
DISCUSSION
The Arabidopsis psd mutation causes delays in a wide range of processes, including leaf production, root elongation, and the vegetative to reproductive transition. In keeping with the pleiotropic nature of the phenotype, we found that PSD is a widely expressed gene coding for the only Arabidopsis ortholog of LOS1/XPOT. This protein has been shown to facilitate the nuclear export of tRNA in both yeast and human systems (Arts et al., 1998a; Kutay et al., 1998; Sarkar and Hopper, 1998). Despite the low level of sequence identity, we have shown that PSD can partially rescue the yeast los1 mutation, suggesting that the ability to mediate tRNA export has been conserved.
Because tRNA export is an essential cellular process, it is surprising that psd mutant plants are viable. Although it is possible that the mutations we have identified still retain some function, we consider this unlikely, because the psd-13 and psd-15 mutations are predicted to delete more than 85% of the protein and disrupt the conserved N-terminal Ran-binding region. It is also unlikely that survival is due to the presence of multiple XPOT genes, because PSD is the only gene in the Arabidopsis genome with significant homology to XPOT (Bollman et al., 2003). The most reasonable interpretation of this result is that Arabidopsis has multiple pathways for tRNA export.
Like psd, null mutations in the single XPOT gene in Brewer's yeast, LOS1, are viable (Hurt et al., 1987), suggesting that there is also at least one additional tRNA export pathway in yeast. Screens for los1 synthetic lethals and multicopy suppressors have led to a model in which the Los1p pathway functions in parallel with a second pathway that requires the nuclear aminoacylation of tRNA before export (for review, see Grosshans et al., 2000b). The two pathways are not fully redundant, because los1 mutations do cause a detectable nuclear accumulation of tRNA, especially of those species encoded by introncontaining genes (Sarkar and Hopper, 1998; Grosshans et al., 2000a). It is also important to note that the relative contribution of the two export mechanisms varies from organism to organism. Xenopus sp. appears to rely primarily on XPOT (Arts et al., 1998b; Lipowsky et al., 1999), whereas fruitfly lacks an XPOT ortholog and must therefore have an alternate mechanism for tRNA export (Lippai et al., 2000).
Recently, XPO5 has been proposed to play a role in mediating tRNA export. The human XPO5 protein has been shown to bind directly to tRNA, and to form a complex with eEF-1A (Bohnsack et al., 2002; Calado et al., 2002), a molecule implicated in the alternate export pathway in yeast (Grosshans et al., 2000a). In addition, XPO5 has been shown to facilitate the export of tRNA from Xenopus sp. oocyte nuclei when the XPOT pathway is saturated (Calado et al., 2002). However, under normal conditions, XPO5 has little stimulatory effect on tRNA export and preferentially binds other dsRNA cargoes (Gwizdek et al., 2002). We found that Arabidopsis plants doubly mutant for the XPOT ortholog PSD and the XPO5 ortholog HST are viable, although reduced in size. It is possible that HST has a different cargo specificity than XPO5; in yeast the XPO5 ortholog, Msn5p has been shown to bind transcription factors (Kaffman et al., 1998a), and msn5 mutants do not affect tRNA export (Feng and Hopper, 2002). It is also possible that Arabidopsis has more than two tRNA export pathways, a model also recently proposed for yeast (Azad et al., 2001; Feng and Hopper, 2002).
Given the general requirement for tRNAs, the effect of psd mutations on specific developmental processes is difficult to explain. One possibility is that PSD is temporally or spatially regulated. Although we have shown that the PSD transcript is present in a variety of tissues and developmental stages, we cannot rule out the transcriptional regulation of PSD at a tissue- or cell-specific level. It is also possible that the translation of PSD is differentially regulated; the 3′-UTR is often the target of molecules that regulate translation (for review, see Gray and Wickens, 1998), and the alternative splicing of the PSD 3′-UTR may control its interaction with such factors. Although we observed no obvious developmental regulation of PSD splicing, it remains possible that PSD splicing and/or translation is altered in response to environmental factors, such as nutrient availability.
The processes affected by psd might also represent developmental events that are particularly sensitive to the loss of the XPOT pathway. Although psd mutations affect many aspects of Arabidopsis development, they have particularly significant effects on major developmental transitions, including the initiation of the SAM, radicle emergence, lateral root initiation, and the transition to flowering. These events may specifically require the XPOT pathway or may require the simultaneous function of all tRNA export pathways to meet translational demands. It is also important to consider the potential for interplay between potential tRNA export pathways. Although XPOT shows a high specificity for tRNA (Arts et al., 1998a; Kutay et al., 1998; Sarkar and Hopper, 1998), XPO5 also binds other dsRNA molecules (Bohnsack et al., 2002; Gwizdek et al., 2002). Developmental defects could result if the loss of XPOT floods alternate pathways with tRNA, disrupting the export of regulatory RNA cargoes, such as miRNA precursors. Further studies of these proteins should increase our understanding of the interactions between RNA export pathways and the functions of karyopherins during development.
MATERIALS AND METHODS
Plant Growth and Culture
Arabidopsis plants of the ecotype Col-0 were grown under constant light at 22°C. To examine root morphology, plants were grown under constant light on 1× Murashige and Skoog salts (Invitrogen, Carlsbad, CA), 2.5 mm MES, 4.5% (w/v) Suc, and 0.9% (w/v) agar (Malamy and Benfey, 1997). Root tissue for RNA extraction was grown in culture in 1/2× Murashige and Skoog salts, 0.02% (w/v) KH2PO4, 0.2 mg mL-1 myo-inositol, and 20% (w/v) Suc, pH 5.7, on a shaker at 22°C under constant light.
Mutagenesis
psd-1 was isolated by ethyl methane sulfonate mutagenesis of Ler as described previously (Barton and Poethig, 1993). This allele was crossed six times into Col before being used for the experiments described here. All other alleles were isolated from fast neutron-mutagenized Col seed. Dry seed were exposed to 60 Gy of fast neutrons at the International Atomic Energy Agency in Vienna and then grown in lots of 500 to 1,000 plants for bulk harvesting. Ten-day-old seedlings from each lot were screened under a dissecting microscope for plants that produced abaxial trichomes at an abnormal leaf position.
Microscopy
psd-6 plants were crossed to Col plants carrying LFY::GUS (gift of D. Weigel) or AP1::GUS (gift of M. Yanofsky). β-Glucoronidase activity was visualized by incubating seedlings overnight in 1 mg mL-1 5-bromo-4-chloro-3-indolyl-β-glucuronic acid, 50 mm sodium phosphate buffer (pH 7), 2 mm potassium ferricyanide, 2 mm potassium ferrocyanide, and 0.1% (v/v) Triton X-100, followed by decolorization in 95% (v/v) ethanol.
To visualize nuclei, seedlings were fixed overnight in a 1:1 (v/v) mixture of hepatane:fix (80 mm EGTA, 5% [v/v] formaldehyde, and 0.1% [v/v] Tween 20 in phosphate-buffered saline) and cleared in 95% (v/v) ethanol. They were then incubated in 70:30 (v/v) ethanol:PBT-0.1 (phosphate-buffered saline and 0.1% [v/v] Tween 20), 50:50 (v/v) ethanol:PBT-0.1, and stained for 10 min in 1 g mL-1 Hoechst 33258 (Sigma-Aldrich, St. Louis) in PBT-0.1. After three washes in PBT-0.1, the seedlings were mounted in Aqua-polymount (Polysciences, Warrington, PA) and imaged using a confocal microscope (TCS-NT, Leica, Wetzlar, Germany). Histological analysis was carried out on seedlings embedded in Spurr's resin, as described previously (Conway and Poethig, 1997).
PSD Cloning
PSD was mapped near nga111 on the bottom of chromosome 1, using an F2 mapping population of 343 plants from a cross between psd-1(Ler) and wild-type Col. BACs from this region were hybridized to genomic DNA from the psd-6 allele, and a HindIII polymorphism was detected using BAC F28P22 as a probe. The corresponding 2-kb HindIII fragment from Col was isolated and used to probe a seedling cDNA library (gift of J. Ecker). Four partial cDNAs corresponding to At1g72560 were isolated and sequenced (GenBank BK001280), and the ends were isolated using the 5′- and 3′-RACE systems (Invitrogen). To locate the mutations in the psd-1, psd-13, and psd-15 alleles, reverse transcription was carried out using Superscript II (Invitrogen), and PCR products were generated using the primers: PSD1B (5′-GTATGCAACAAACCAGGACTGG-3′); PSD5862 (5′-CTCTCCGATGAAGATTCAACAGC-3′); PSD5758 (5′-AGATCTCTTTGTCTTATTACG-3′); PSD583 (5′-ATCATGGAGAGCGCTGTTGAA-3′); PSD7431 (5′-CCCAAGGCTTTGGAACAGCTAC-3′); and PSD9417 (5′-CATCGACTGCAGAGAGTTATAAG-3′). The products of these reactions were gel isolated using the QIAquick gel extraction kit (Qiagen USA, Valencia, CA) before sequencing.
psd Complementation
A 7.6-kb SalI-PstI genomic fragment containing the PSD predicted open reading frame plus 2.8 kb of upstream sequence and 300 bp of downstream sequence was isolated from the BAC T16D11 and subcloned into the SalI and PstI sites of pCAMBIA2300 (CAMBIA). As a negative control, a BamHI fragment containing 4.4 kb of upstream DNA and the first 1 kb of the PSD open reading frame was subcloned into the HindIII site of pCAMBIA2300. Both constructs were transformed into Agrobacterium tumefaciens strain GV3101 pMP90 by electroporation. psd-6 mutant plants were transformed by the floral dip method (Clough and Bent, 1998) and selected on plates (1/4× Murashige and Skoog salts, 3% [w/v] bactoagar, and 75 mg L-1 kanamycin sulfate).
Expression Analysis
Leaf and floral bud tissues were isolated from mature plants, and root tissue was grown in culture (see above). Eight-, 15-, and 22-d shoot apices were obtained by removing the roots and all visible leaves from the shoots of Col plants grown under short-day conditions (8 h of light and 16 h of dark). RNA was prepared using TRIzol reagent (Invitrogen), and poly(A) RNA was isolated using the Oligotex mRNA kit (Qiagen USA). Poly(A) RNA (0.75 μg) was run on a 1.2% (w/v) agarose gel containing 3% (v/v) formaldehyde and was transferred to Hybond N+ membrane (Amersham Biosciences, Uppsala). Probes to PSD were generated by combining RT-PCR products amplified with the PSD1B-PSD1C (5′-TAGCCAAAACCTGTGCAAGC-3′), PSD5758-PSD583, and PSD7431-PSD9417 primer pairs. Actin probes were generated using the primers 5′-AAGATGACCCAAATCATGTTTGAGAC-3′ and 5′-ACGACCTTGATCTTCATGCTGC-3′. Probes were labeled using the Prime-it II kit (Stratagene, La Jolla, CA), and hybridization was carried out as described by Smith et al. (1992).
For analysis of the expression of alternatively spliced transcripts, RNA from the above tissues was reverse transcribed, and PCR was carried out for 10, 15, 20, or 25 cycles using a combination of primers PSD8947 (5′-GACTAGTGTCGACCGGGAGTCACGTTTTCAGATAG-3′) and PSD9417 at a 1 μm concentration, and the primers ACT8F (5′-GCCAGATCTTCATCGTCGTGGTTCTTG-3′) and ACT8R (5′-CCAATGGCGTAAAGTGATAGAACAGC-3′) at a 0.125 μm concentration. The resulting products were digested with DdeI to distinguish the 13A and 13B forms, and the digests were run on a 4% (w/v) MetaPhor gel (BMA Biomedicals, Augst, Switzerland) and transferred to Hybond N+ membrane (Amersham Biosciences). Probes were generated using the same primer sets, and hybridization was carried out by standard methods (Sambrook et al., 1989).
los1 Complementation
A 3.5-kb HpaI-SalI fragment containing the PSD open reading frame was ligated to the SmaI and SalI sites of the p426ADH vector (ATCC). The ligation mixture was transformed into the Brewer's yeast strain X2316 (MATαSUP4 his5-2 lys1-1 can1-100 trp5-48 ade2-1 ura3-1; gift of A. Hopper) transformants were selected on synthetic dextrose complete (SDC) (-ura) plates and were screened for the insertion using the PSD-specific primers PSD469 (5′-GTCTTTACTGCTTACCCCTCT-3′) and PSD583. Plasmid DNA was isolated from selected transformants and was used to transform the strain 201-1-5 (MATαSUP4 his5-2 lys1-1 can1-100 trp5-48 ade2-1 ura3-1 los1-1; gift of A. Hopper). X2316-3C, 201-1-5, and 201-1-5 [PSD+] were grown at permissive temperature (22°C) to stationary phase, serially diluted 1:5 (v/v) in water, plated on SDC (-ade), and grown at permissive or restrictive (34°C) temperature. No growth was seen in controls containing p426 alone or in [URA-] revertants of the [PSD+] transformants.
Distribution of Materials
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third-party owners of all or parts of the material. Obtaining any permissions will be the responsibility of the requestor.
Acknowledgments
We are grateful to Randy Kerstetter, Tanya Berardini, and Matt Sauer for advice and helpful discussions and to Kathy Tworkowski for technical assistance. We also thank Joe Ecker, Anita Hopper, Marty Yanofsky, and Detlef Weigel for reagents used in this study.
This work was supported by the National Institutes of Health (grants to C.A.H. and R.S.P.).
References
- Arts GJ, Fornerod M, Mattaj IW (1998a) Identification of a nuclear export receptor for tRNA. Curr Biol 8: 305-314 [DOI] [PubMed] [Google Scholar]
- Arts GJ, Kuersten S, Romby P, Ehresmann B, Mattaj IW (1998b) The role of exportin-t in selective nuclear export of mature tRNAs. EMBO J 17: 7430-7441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad AK, Stanford DR, Sarkar S, Hopper AK (2001) Role of nuclear pools of aminoacyl-tRNA synthetases in tRNA nuclear export. Mol Biol Cell 12: 1381-1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton MK, Poethig RS (1993) Formation of the shoot apical meristem in Arabidopsis thaliana and analysis of development in the wild type and in the shoot meristemless mutant. Development 119: 823-831 [Google Scholar]
- Blazquez MA, Soowal LN, Lee I, Weigel D (1997) LEAFY expression and flower initiation in Arabidopsis. Development 124: 3835-3844 [DOI] [PubMed] [Google Scholar]
- Bohnsack MT, Regener K, Schwappach B, Saffrich R, Paraskeva E, Hartmann E, Gorlich D (2002) Exp5 exports eEF1A via tRNA from nuclei and synergizes with other transport pathways to confine translation to the cytoplasm. EMBO J 21: 6205-6215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollman KM, Aukerman MJ, Park MY, Hunter C, Berardini TZ, Poethig RS (2003) HASTY, the Arabidopsis ortholog of exportin 5/MSN5, regulates phase change and morphogenesis. Development 130: 1493-1504 [DOI] [PubMed] [Google Scholar]
- Calado A, Treichel N, Muller EC, Otto A, Kutay U (2002) Exportin-5-mediated nuclear export of eukaryotic elongation factor 1A and tRNA. EMBO J 21: 6216-6224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735-743 [DOI] [PubMed] [Google Scholar]
- Collier S, Chan HY, Toda T, McKimmie C, Johnson G, Adler PN, O'Kane C, Ashburner M (2000) The Drosophila embargoed gene is required for larval progression and encodes the functional homolog of Schizosaccharomyces Crm1. Genetics 155: 1799-1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway LJ, Poethig RS (1997) Mutations of Arabidopsis thaliana that transform leaves into cotyledons. Proc Natl Acad Sci USA 94: 10209-10214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo MC, Crawford ME, Hirschman JE, Kranz JE, Olsen P, Robertson LS, Skrzypek MS, Braun BR, Hopkins KL, Kondu P et al. (2001) YPD, PombePD and WormPD: model organism volumes of the BioKnowledge library, an integrated resource for protein information. Nucleic Acids Res 29: 75-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W, Hopper AK (2002) A Los1p-independent pathway for nuclear export of intronless tRNAs in Saccharomyces cerevisiae. Proc Natl Acad Sci USA 99: 5412-5417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray NK, Wickens M (1998) Control of translation initiation in animals. Annu Rev Cell Dev Biol 14: 399-458 [DOI] [PubMed] [Google Scholar]
- Grosshans H, Hurt E, Simos G (2000a) An aminoacylation-dependent nuclear tRNA export pathway in yeast. Genes Dev 14: 830-840 [PMC free article] [PubMed] [Google Scholar]
- Grosshans H, Simos G, Hurt E (2000b) Transport of tRNA out of the nucleus-direct channeling to the ribosome? J Struct Biol 129: 288-294 [DOI] [PubMed] [Google Scholar]
- Gwizdek C, Ossareh-Nazari B, Brownawell AM, Doglio A, Bertrand E, Macara IG, Dargemont C (2002) Exportin-5 mediates nuclear export of minihelix-containing RNAs. J Biol Chem 30: 30. [DOI] [PubMed] [Google Scholar]
- Haasen D, Kohler C, Neuhaus G, Merkle T (1999) Nuclear export of proteins in plants: AtXPO1 is the export receptor for leucine-rich nuclear export signals in Arabidopsis thaliana. Plant J 20: 695-705 [DOI] [PubMed] [Google Scholar]
- Haasen D, Merkle T (2002) Characterization of an Arabidopsis thaliana homologue of the nuclear export receptor CAS by its interaction with importin alpha. Plant Biol 4: 432-439 [Google Scholar]
- Hempel FD, Weigel D, Mandel MA, Ditta G, Zambryski PC, Feldman LJ, Yanofsky MF (1997) Floral determination and expression of floral regulatory genes in Arabidopsis. Development 124: 3845-3853 [DOI] [PubMed] [Google Scholar]
- Hopper AK, Schultz LD, Shapiro RA (1980) Processing of intervening sequences: a new yeast mutant which fails to excise intervening sequences from precursor tRNAs. Cell 19: 741-751 [DOI] [PubMed] [Google Scholar]
- Hurt DJ, Wang SS, Lin YH, Hopper AK (1987) Cloning and characterization of LOS1, a Saccharomyces cerevisiae gene that affects tRNA splicing. Mol Cell Biol 7: 1208-1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaffman A, Rank NM, O'Neill EM, Huang LS, O'Shea EK (1998a) The receptor Msn5 exports the phosphorylated transcription factor Pho4 out of the nucleus. Nature 396: 482-486 [DOI] [PubMed] [Google Scholar]
- Kaffman A, Rank NM, O'Shea EK (1998b) Phosphorylation regulates association of the transcription factor Pho4 with its import receptor Pse1/Kap121. Genes Dev 12: 2673-2683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutay U, Lipowsky G, Izaurralde E, Bischoff FR, Schwarzmaier P, Hartmann E, Gorlich D (1998) Identification of a tRNA-specific nuclear export receptor. Mol Cell 1: 359-369 [DOI] [PubMed] [Google Scholar]
- Lipowsky G, Bischoff FR, Izaurralde E, Kutay U, Schafer S, Gross HJ, Beier H, Gorlich D (1999) Coordination of tRNA nuclear export with processing of tRNA. RNA 5: 539-549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippai M, Tirian L, Boros I, Mihaly J, Erdelyi M, Belecz I, Mathe E, Posfai J, Nagy A, Udvardy A et al. (2000) The Ketel gene encodes a Drosophila homologue of importin-beta. Genetics 156: 1889-1900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzen JA, Baker SE, Denhez F, Melnick MB, Brower DL, Perkins LA (2001) Nuclear import of activated D-ERK by DIM-7, an importin family member encoded by the gene moleskin. Development 128: 1403-1414 [DOI] [PubMed] [Google Scholar]
- Malamy JE, Benfey PN (1997) Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124: 33-44 [DOI] [PubMed] [Google Scholar]
- Nakielny S, Dreyfuss G (1999) Transport of proteins and RNAs in and out of the nucleus. Cell 99: 677-690 [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual, Ed 2. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Sarkar S, Hopper AK (1998) tRNA nuclear export in Saccharomyces cerevisiae: in situ hybridization analysis. Mol Biol Cell 9: 3041-3055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LG, Greene B, Veit B, Hake S (1992) A dominant mutation in the maize homeobox gene, Knotted-1, causes its ectopic expression in leaf cells with altered fates. Development 116: 21-30 [DOI] [PubMed] [Google Scholar]
- Steeves TA, Sussex IM (1989) Patterns in Plant Development, Ed 2. Cambridge University Press, Cambridge
- Stein L, Sternberg P, Durbin R, Thierry-Mieg J, Spieth J (2001) WormBase: network access to the genome and biology of Caenorhabditis elegans. Nucleic Acids Res 29: 82-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telfer A, Bollman KM, Poethig RS (1997) Phase change and the regulation of trichome distribution in Arabidopsis thaliana. Development 124: 645-654 [DOI] [PubMed] [Google Scholar]
- Tirian L, Puro J, Erdelyi M, Boros I, Papp B, Lippai M, Szabad J (2000) The Ketel(D) dominant-negative mutations identify maternal function of the Drosophila importin-beta gene required for cleavage nuclei formation. Genetics 156: 1901-1912 [DOI] [PMC free article] [PubMed] [Google Scholar]