Abstract
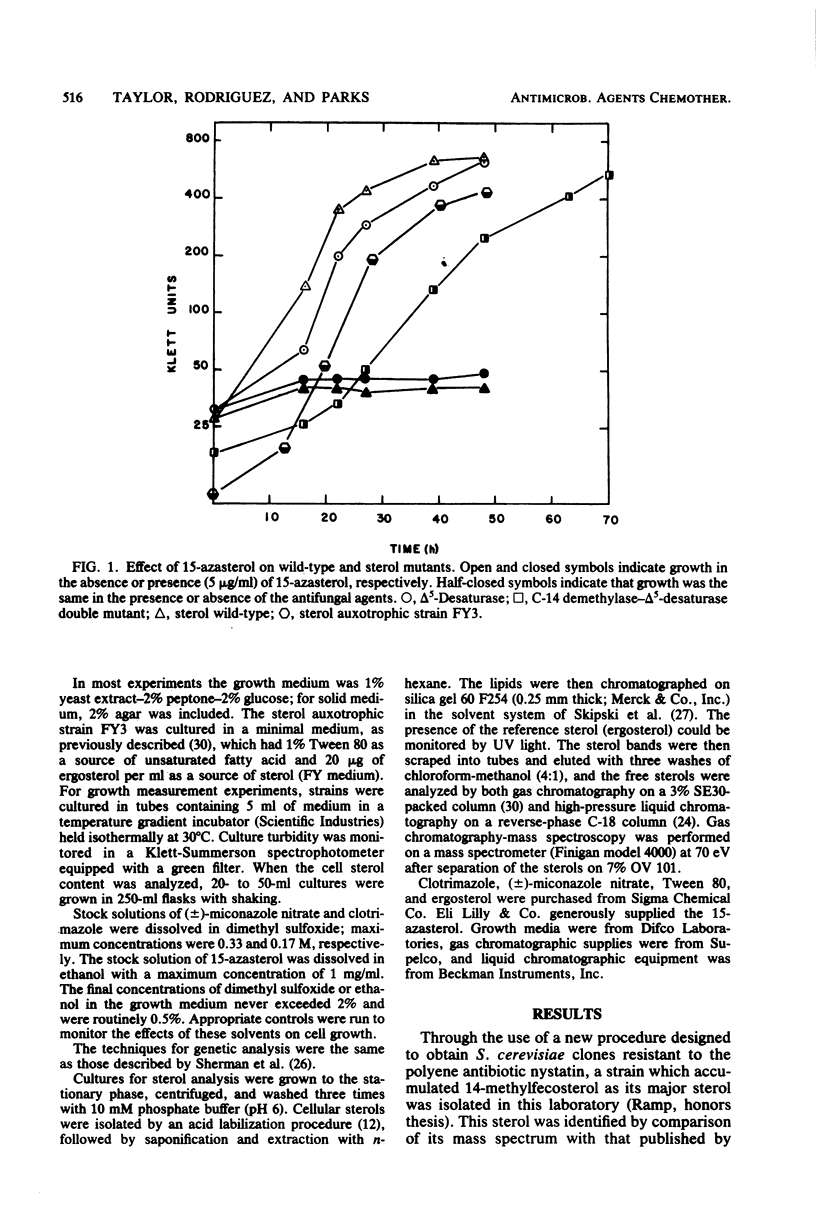
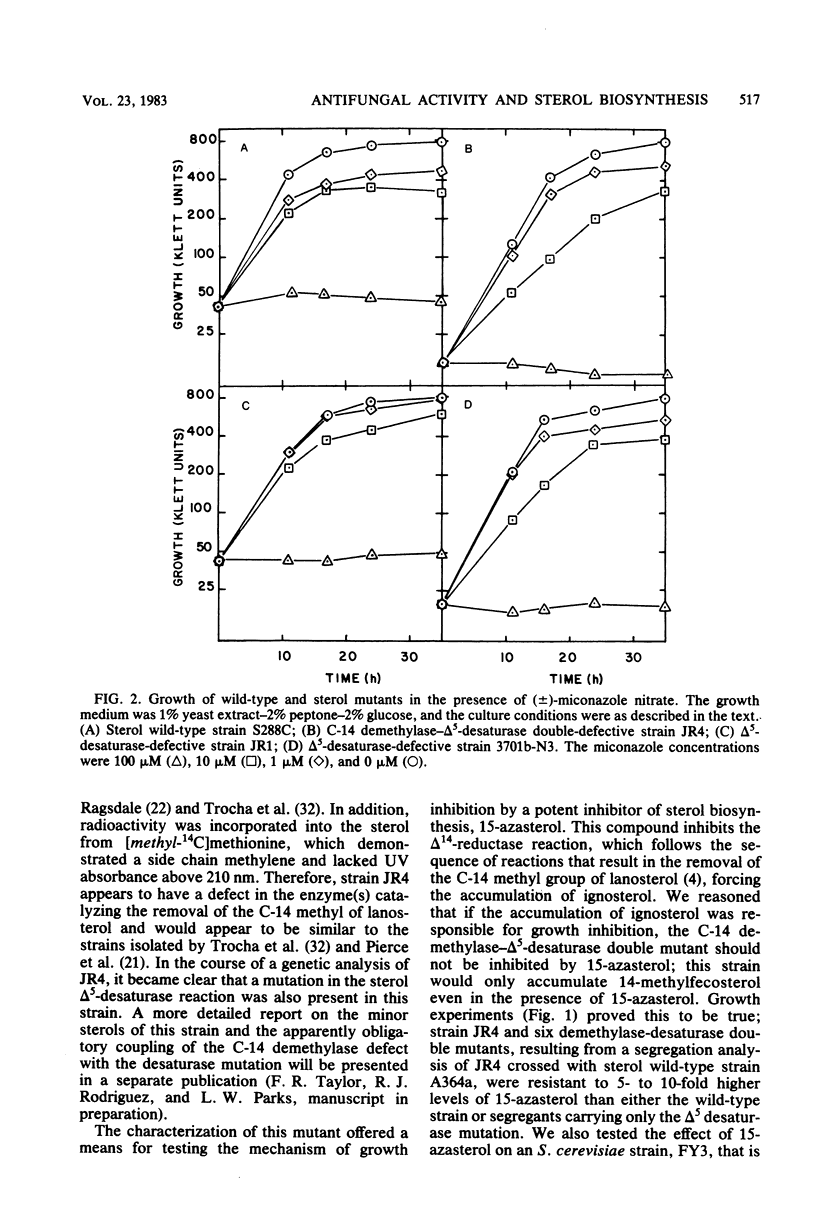
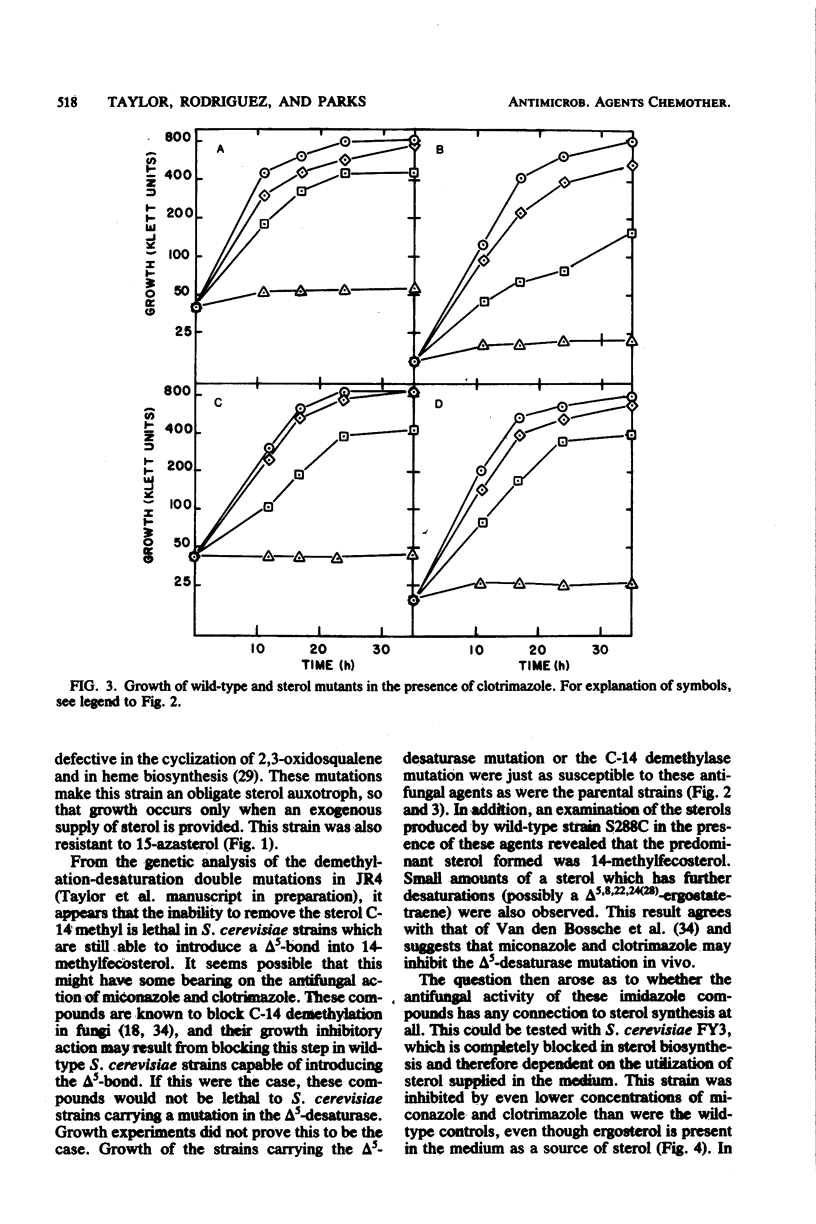
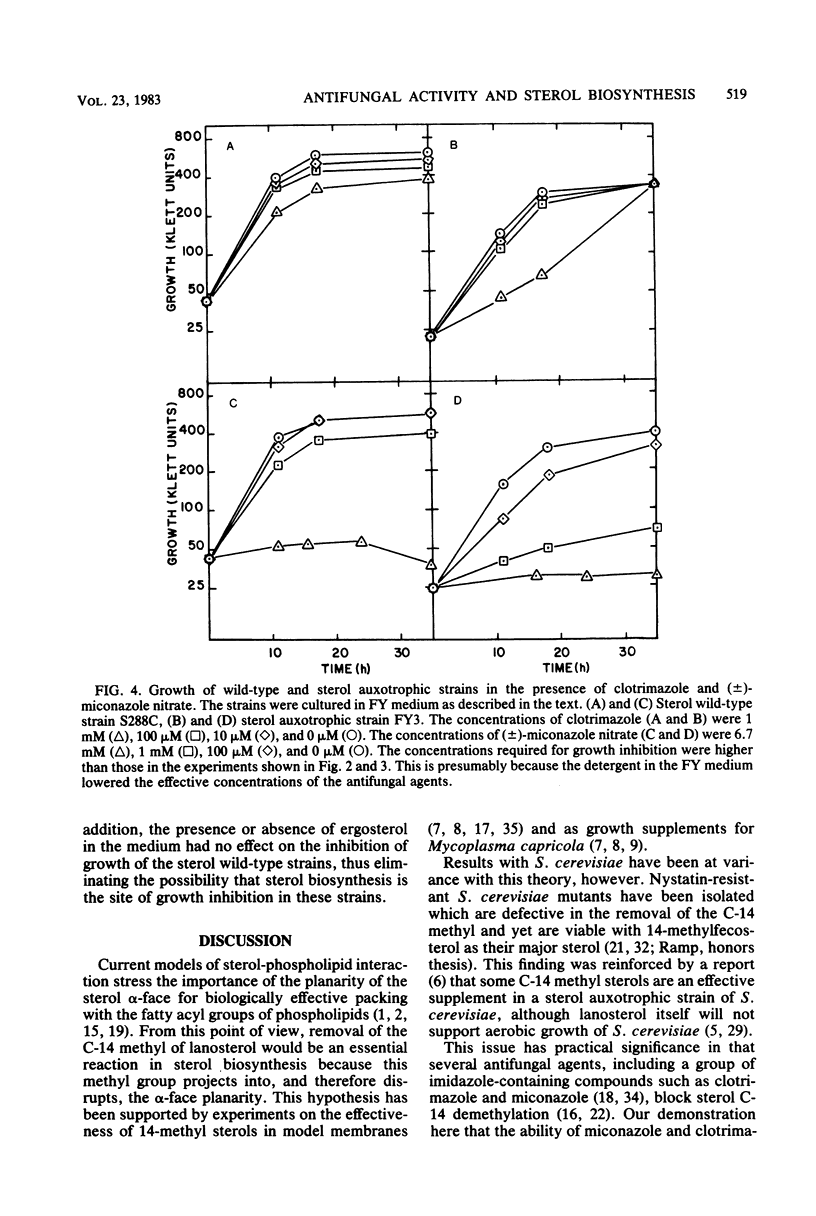
The availability of Saccharomyces cerevisiae mutants which are defective in sterol biosynthesis makes it possible to determine whether the ability of several antifungal agents to inhibit cell growth is due to their effect on sterol production. 15-Aza-24-methylene-8,14-cholestadien-3 beta-ol (15-azasterol) is known to block the reduction of the sterol delta 14 bond following C-14 demethylation. This agent inhibits the growth of wild-type S. cerevisiae but does not inhibit the growth of a strain that is defective in the removal of the C-14 methyl group of lanosterol and in the introduction of the 5,6 double bond. 15-Azasterol does not inhibit the growth of a sterol auxotrophic strain growing on an exogenous supply of sterol. Therefore, the effect of 15-azasterol on sterol biosynthesis is clearly the cause of its ability to inhibit growth. On the other hand, growth inhibition by two imidazole antifungal agents, clotrimazole and miconazole, cannot be ascribed to their ability to prevent the removal of the C-14 methyl group of lanosterol, because they inhibit the growth of the sterol auxotrophic strain as well as that of the demethylase mutant.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloch K. Sterol structure and membrane function. Curr Top Cell Regul. 1981;18:289–299. doi: 10.1016/b978-0-12-152818-8.50022-0. [DOI] [PubMed] [Google Scholar]
- Bottema C. K., Parks L. W. Delta14-sterol reductase in Saccharomyces cerevisiae. Biochim Biophys Acta. 1978 Dec 22;531(3):301–307. doi: 10.1016/0005-2760(78)90212-6. [DOI] [PubMed] [Google Scholar]
- Buttke T. M., Bloch K. Comparative responses of the yeast mutant strain GL7 to lanosterol, cycloartenol, and cyclolaudenol. Biochem Biophys Res Commun. 1980 Jan 15;92(1):229–236. doi: 10.1016/0006-291x(80)91543-0. [DOI] [PubMed] [Google Scholar]
- Buttke T. M., Bloch K. Utilization and metabolism of methyl-sterol derivatives in the yeast mutant strain GL7. Biochemistry. 1981 May 26;20(11):3267–3272. doi: 10.1021/bi00514a044. [DOI] [PubMed] [Google Scholar]
- Dahl C. E., Dahl J. S., Bloch K. Effect of alkyl-substituted precursors of cholesterol on artificial and natural membranes and on the viability of Mycoplasma capricolum. Biochemistry. 1980 Apr 1;19(7):1462–1467. doi: 10.1021/bi00548a031. [DOI] [PubMed] [Google Scholar]
- Dahl C. E., Dahl J. S., Bloch K. Effects of cycloartenol and lanosterol on artificial and natural membranes. Biochem Biophys Res Commun. 1980 Jan 15;92(1):221–228. doi: 10.1016/0006-291x(80)91542-9. [DOI] [PubMed] [Google Scholar]
- Dahl J. S., Dahl C. E., Bloch K. Effect of cholesterol on macromolecular synthesis and fatty acid uptake by Mycoplasma capricolum. J Biol Chem. 1981 Jan 10;256(1):87–91. [PubMed] [Google Scholar]
- Dahl J. S., Dahl C. E., Bloch K. Sterols in membranes: growth characteristics and membrane properties of Mycoplasma capricolum cultured on cholesterol and lanosterol. Biochemistry. 1980 Apr 1;19(7):1467–1472. doi: 10.1021/bi00548a032. [DOI] [PubMed] [Google Scholar]
- Dufour J. P., Boutry M., Goffeau A. Plasma membrane ATPase of yeast. Comparative inhibition studies of the purified and membrane-bound enzymes. J Biol Chem. 1980 Jun 25;255(12):5735–5741. [PubMed] [Google Scholar]
- Gonzales R. A., Parks L. W. Acid-labilization of sterols for extraction from yeast. Biochim Biophys Acta. 1977 Dec 21;489(3):507–509. doi: 10.1016/0005-2760(77)90171-0. [DOI] [PubMed] [Google Scholar]
- Hays P. R., Neal W. D., Parks L. W. Physiological effects of an antimycotic azasterol on cultures of Saccharomyces cerevisiae. Antimicrob Agents Chemother. 1977 Aug;12(2):185–191. doi: 10.1128/aac.12.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays P. R., Parks L. W., Pierce H. D., Jr, Oehlschlager A. C. Accumulation of ergosta-8,14-dien-3beta-ol by Saccharomyces cerevisiae cultured with an azasterol antimycotic agent. Lipids. 1977 Aug;12(8):666–668. doi: 10.1007/BF02533762. [DOI] [PubMed] [Google Scholar]
- Huang C. Configurations of fatty acyl chains in egg phosphatidylcholine-cholesterol mixed bilayers. Chem Phys Lipids. 1977 Jun;19(2):150–158. doi: 10.1016/0009-3084(77)90095-0. [DOI] [PubMed] [Google Scholar]
- Marriott M. S. Inhibition of sterol biosynthesis in Candida albicans by imidazole-containing antifungals. J Gen Microbiol. 1980 Mar;117(1):253–255. doi: 10.1099/00221287-117-1-253. [DOI] [PubMed] [Google Scholar]
- Parks L. W. Metabolism of sterols in yeast. CRC Crit Rev Microbiol. 1978;6(4):301–341. doi: 10.3109/10408417809090625. [DOI] [PubMed] [Google Scholar]
- Ragsdale N. N. Specific effects of triarimol on sterol biosynthesis in Ustilago maydis. Biochim Biophys Acta. 1975 Jan 24;380(1):81–96. doi: 10.1016/0005-2760(75)90047-8. [DOI] [PubMed] [Google Scholar]
- Rodriguez R. J., Parks L. W. Application of high-performance liquid chromatographic separation of free sterols to the screening of yeast sterol mutants. Anal Biochem. 1982 Jan 1;119(1):200–204. doi: 10.1016/0003-2697(82)90686-8. [DOI] [PubMed] [Google Scholar]
- Rodriguez R. J., Parks L. W. Physiological response of Saccharomyces cerevisiae to 15-azasterol-mediated growth inhibition. Antimicrob Agents Chemother. 1981 Aug;20(2):184–189. doi: 10.1128/aac.20.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez R. J., Taylor F. R., Parks L. W. A requirement for ergosterol to permit growth of yeast sterol auxotrophs on cholestanol. Biochem Biophys Res Commun. 1982 May 31;106(2):435–441. doi: 10.1016/0006-291x(82)91129-9. [DOI] [PubMed] [Google Scholar]
- Skipski V. P., Smolowe A. F., Sullivan R. C., Barclay M. Separation of lipid classes by thin-layer chromatography. Biochim Biophys Acta. 1965 Oct 4;106(2):386–396. doi: 10.1016/0005-2760(65)90047-0. [DOI] [PubMed] [Google Scholar]
- Sud I. J., Feingold D. S. Heterogeneity of action of mechanisms among antimycotic imidazoles. Antimicrob Agents Chemother. 1981 Jul;20(1):71–74. doi: 10.1128/aac.20.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor F. R., Parks L. W. Adaptation of Saccharomyces cerevisiae to growth on cholesterol: selection of mutants defective in the formation of lanosterol. Biochem Biophys Res Commun. 1980 Aug 29;95(4):1437–1445. doi: 10.1016/s0006-291x(80)80058-1. [DOI] [PubMed] [Google Scholar]
- Taylor F. R., Parks L. W. An assessment of the specificity of sterol uptake and esterification in Saccharomyces cerevisiae. J Biol Chem. 1981 Dec 25;256(24):13048–13054. [PubMed] [Google Scholar]
- Thompson E. D., Parks L. W. The effect of altered sterol composition on cytochrome oxidase and S-adenosylmethionine: delta 24 sterol methyltransferase enzymes of yeast mitochondria. Biochem Biophys Res Commun. 1974 Apr 23;57(4):1207–1213. doi: 10.1016/0006-291x(74)90825-0. [DOI] [PubMed] [Google Scholar]
- Trocha P. J., Jasne S. J., Sprinson D. B. Yeast mutants blocked in removing the methyl group of lanosterol at C-14. Separation of sterols by high-pressure liquid chromatography. Biochemistry. 1977 Oct 18;16(21):4721–4726. doi: 10.1021/bi00640a029. [DOI] [PubMed] [Google Scholar]
- Van den Bossche H., Willemsens G., Cools W., Cornelissen F., Lauwers W. F., van Cutsem J. M. In vitro and in vivo effects of the antimycotic drug ketoconazole on sterol synthesis. Antimicrob Agents Chemother. 1980 Jun;17(6):922–928. doi: 10.1128/aac.17.6.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeagle P. L., Martin R. B., Lala A. K., Lin H. K., Bloch K. Differential effects of cholesterol and lanosterol on artificial membranes. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4924–4926. doi: 10.1073/pnas.74.11.4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bossche H., Willemsens G., Cools W., Lauwers W. F., Le Jeune L. Biochemical effects of miconazole on fungi. II. Inhibition of ergosterol biosynthesis in Candida albicans. Chem Biol Interact. 1978 Apr;21(1):59–78. doi: 10.1016/0009-2797(78)90068-6. [DOI] [PubMed] [Google Scholar]


