Abstract
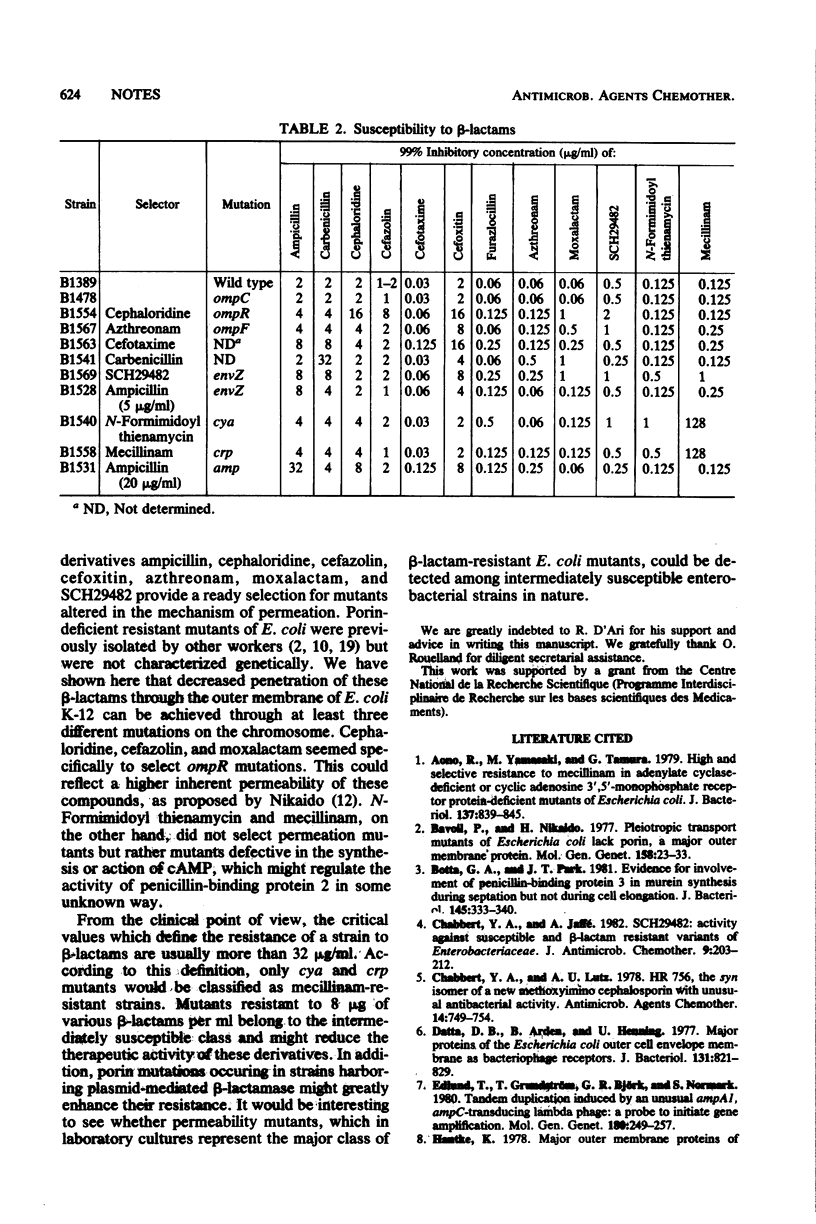
beta-Lactam-resistant mutants of Escherichia coli K-12 were selected by using 12 different beta-lactam derivatives. The mutants fell into three categories showing (i) altered permeation through reduction or loss of outer membrane porin proteins (including ompF, ompR, and envZ alleles); (ii) increase in the rate of synthesis of chromosomally mediated beta-lactamase; or (iii) defective synthesis or action of cyclic adenosine 3',5'-phosphate (cya and crp alleles).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chabbert Y. A., Jaffé A. Sch 29482: activity against susceptible and beta-lactam resistant variants of Enterobacteriaceae. J Antimicrob Chemother. 1982 Feb;9 (Suppl 100):203–212. doi: 10.1093/jac/9.suppl_c.203. [DOI] [PubMed] [Google Scholar]
- Harder K. J., Nikaido H., Matsuhashi M. Mutants of Escherichia coli that are resistant to certain beta-lactam compounds lack the ompF porin. Antimicrob Agents Chemother. 1981 Oct;20(4):549–552. doi: 10.1128/aac.20.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe A., Chabbert Y. A., Semonin O. Role of porin proteins OmpF and OmpC in the permeation of beta-lactams. Antimicrob Agents Chemother. 1982 Dec;22(6):942–948. doi: 10.1128/aac.22.6.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Nakae T. The outer membrane of Gram-negative bacteria. Adv Microb Physiol. 1979;20:163–250. doi: 10.1016/s0065-2911(08)60208-8. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G., Pardee A. B. Penicillin-binding proteins and cell shape in E. coli. Nature. 1975 Apr 10;254(5500):516–517. doi: 10.1038/254516a0. [DOI] [PubMed] [Google Scholar]
- Sykes R. B., Bonner D. P., Bush K., Georgopapadakou N. H. Azthreonam (SQ 26,776), a synthetic monobactam specifically active against aerobic gram-negative bacteria. Antimicrob Agents Chemother. 1982 Jan;21(1):85–92. doi: 10.1128/aac.21.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. K., Hall M. N., Enquist L., Silhavy T. J. Identification of OmpR: a positive regulatory protein controlling expression of the major outer membrane matrix porin proteins of Escherichia coli K-12. J Bacteriol. 1981 Jul;147(1):255–258. doi: 10.1128/jb.147.1.255-258.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandersman C., Moreno F., Schwartz M. Pleiotropic mutations rendering Escherichia coli K-12 resistant to bacteriophage TP1. J Bacteriol. 1980 Sep;143(3):1374–1383. doi: 10.1128/jb.143.3.1374-1383.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandersman C., Schwartz M. Protein Ia and the lamB protein can replace each other in the constitution of an active receptor for the same coliphage. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5636–5639. doi: 10.1073/pnas.75.11.5636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R., Andrews J. M., Bedford K. A. Comparison of the in vitro activity of Bay k 4999 and piperacillin, two new antipseudomonal broad-spectrum penicillins, with other beta-lactam drugs. Antimicrob Agents Chemother. 1978 Oct;14(4):549–552. doi: 10.1128/aac.14.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]


