Abstract
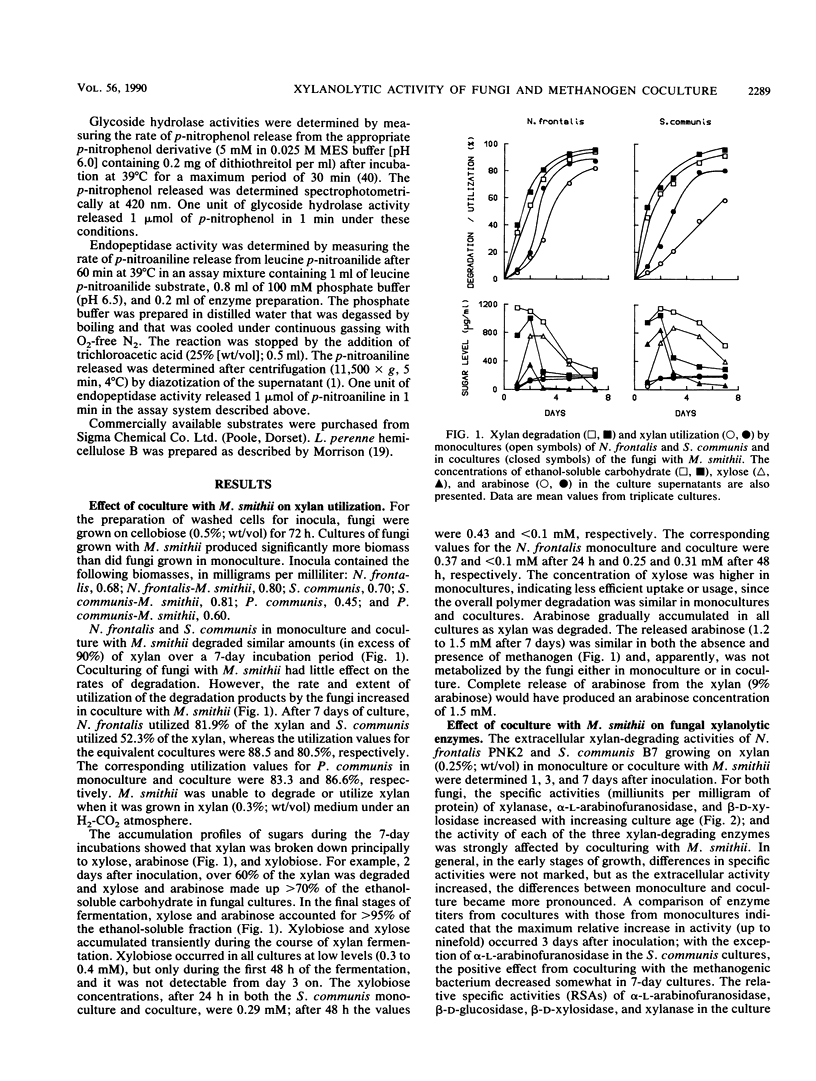
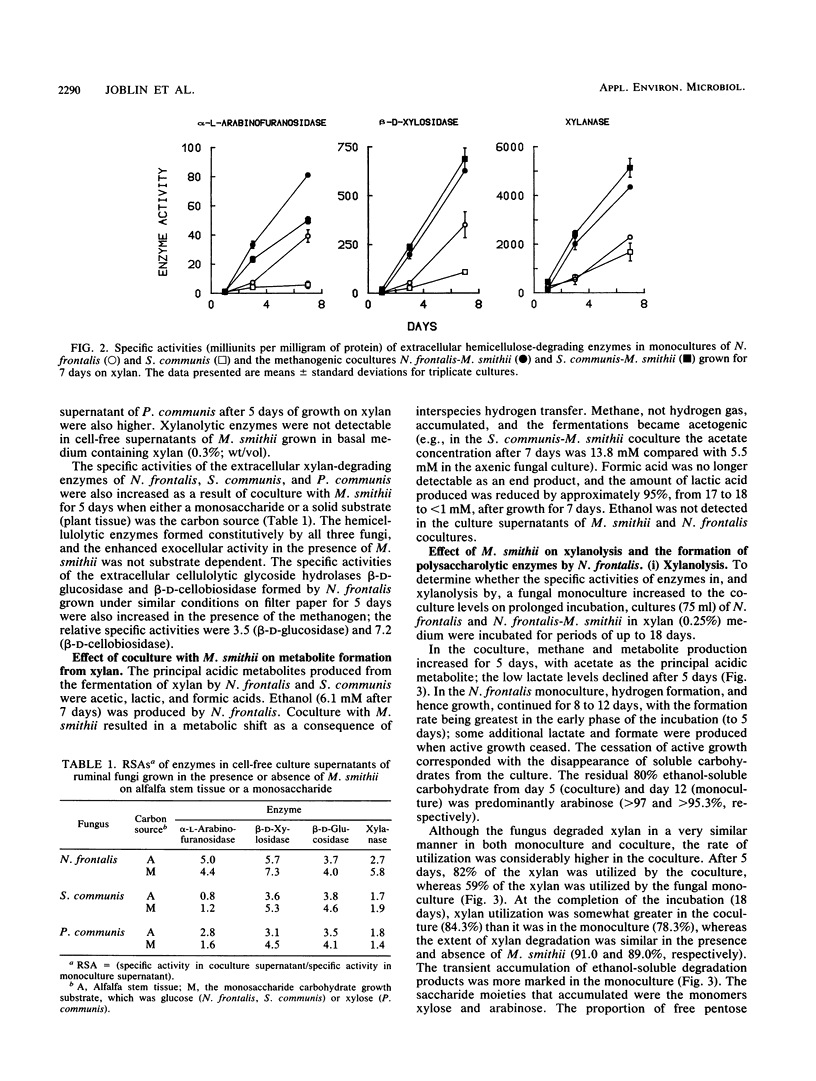
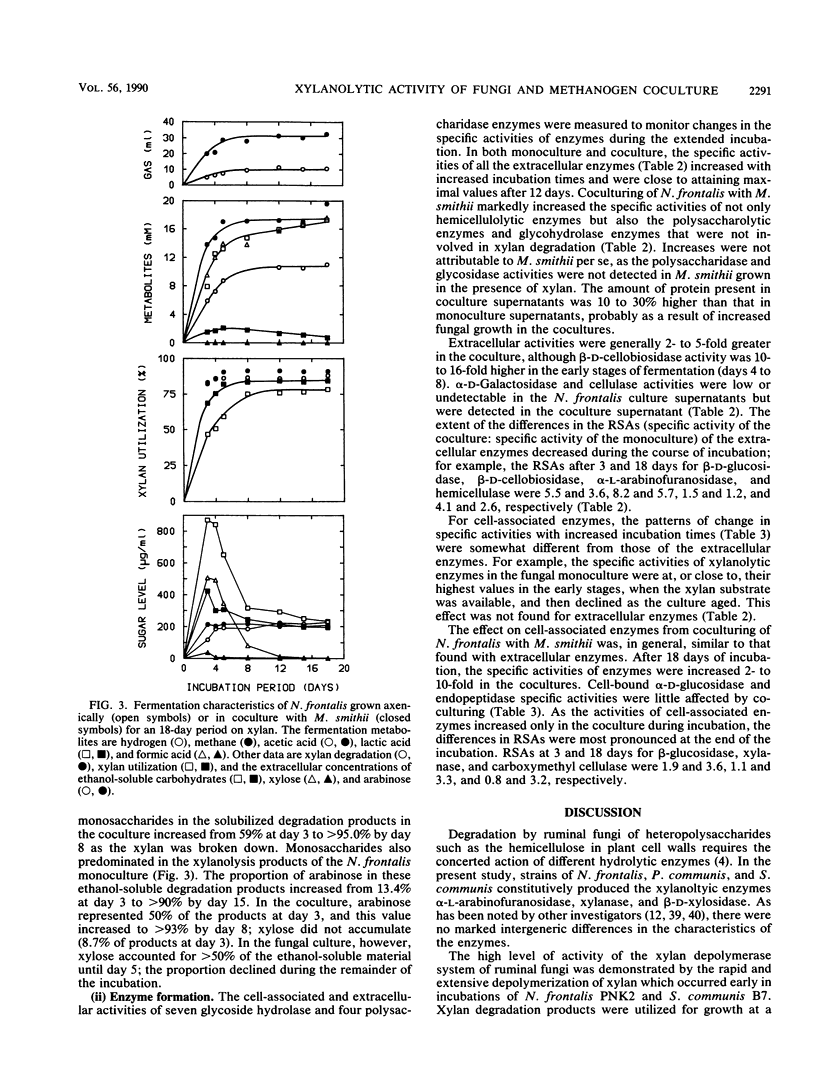
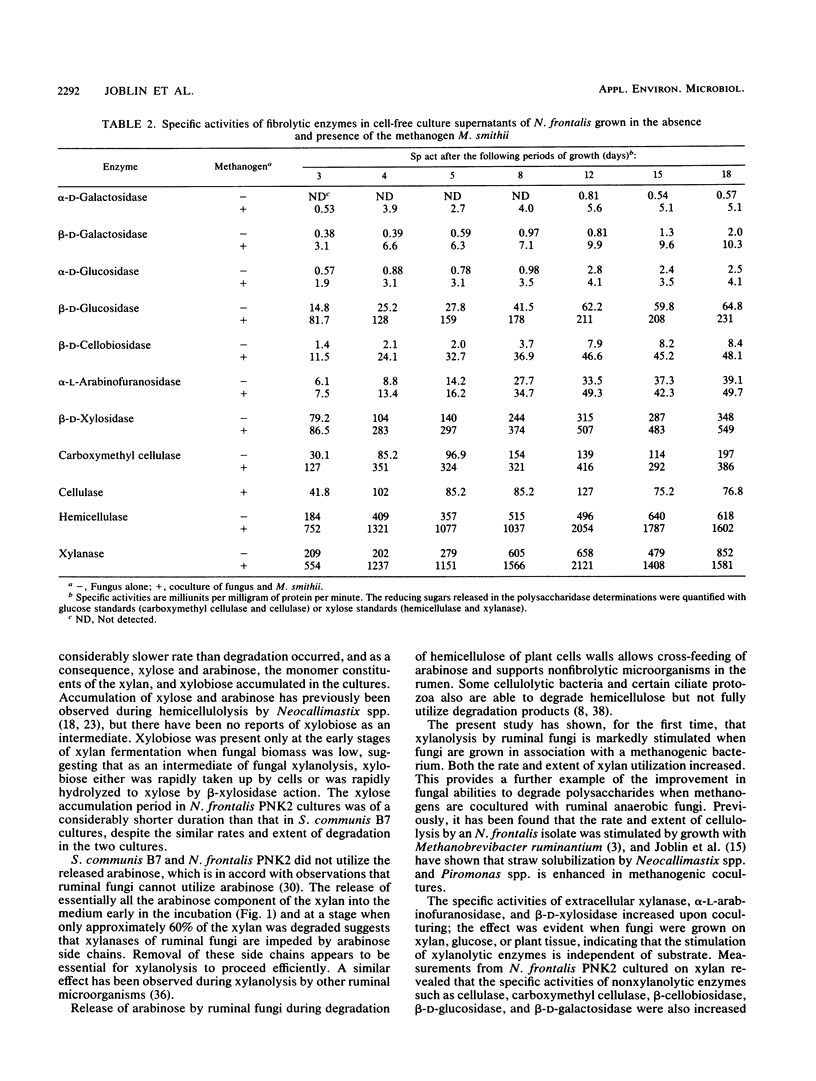
Three different ruminal anaerobic fungi, Neocallimastix frontalis PNK2, Sphaeromonas communis B7, and Piromonas communis B19, were grown axenically or in coculture with Methanobrevibacter smithii on xylan. N. frontalis and S. communis in monoculture and coculture accumulated xylobiose, xylose, and arabinose in the growth medium; arabinose was not metabolized, but xylobiose and xylose were subsequently used. The transient accumulation of xylose was much less evident in cocultures. Both the rate and extent of xylan utilization were increased by coculturing, and metabolite profiles became acetogenic as a result of interspecies hydrogen transfer; more acetate and less lactate were formed, while formate and hydrogen did not accumulate. For each of the three fungi, there were marked increases in the specific activities of extracellular xylanase (up to fivefold), α-l-arabinofuranosidase (up to fivefold), and β-d-xylosidase (up to sevenfold) upon coculturing. The stimulating effect on fungal enzymes from coculturing with M. smithii was independent of the growth substrate, and the magnitude of the stimulation varied according to the enzymes and the incubation time. For an N. frontalis-M. smithii coculture, the positive stimulation was maintained during an extended (18-day) incubation period, and this affected not only hemicellulolytic enzymes but also polysaccharidase and glycoside hydrolase enzymes that were not involved in xylan breakdown. The specific activity of cell-bound endopeptidase was not increased under the coculture conditions used in this study. The higher enzyme activities in cocultures are discussed in relation to catabolite repression.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauchop T., Mountfort D. O. Cellulose fermentation by a rumen anaerobic fungus in both the absence and the presence of rumen methanogens. Appl Environ Microbiol. 1981 Dec;42(6):1103–1110. doi: 10.1128/aem.42.6.1103-1110.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borneman W. S., Akin D. E., Ljungdahl L. G. Fermentation products and plant cell wall-degrading enzymes produced by monocentric and polycentric anaerobic ruminal fungi. Appl Environ Microbiol. 1989 May;55(5):1066–1073. doi: 10.1128/aem.55.5.1066-1073.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- DEHORITY B. A. DEGRADATION AND UTILIZATION OF ISOLATED HEMICELLULOSE BY PURE CULTURES OF CELLULOLYTIC RUMEN BACTERIA. J Bacteriol. 1965 Jun;89:1515–1520. doi: 10.1128/jb.89.6.1515-1520.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehority B. A. Hemicellulose degradation by rumen bacteria. Fed Proc. 1973 Jul;32(7):1819–1825. [PubMed] [Google Scholar]
- Joblin K. N. Isolation, enumeration, and maintenance of rumen anaerobic fungi in roll tubes. Appl Environ Microbiol. 1981 Dec;42(6):1119–1122. doi: 10.1128/aem.42.6.1119-1122.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever M. Carbohydrate determination with 4-hydroxybenzoic acid hydrazide (PAHBAH): effect of bismuth on the reaction. Anal Biochem. 1977 Jul;81(1):21–27. doi: 10.1016/0003-2697(77)90594-2. [DOI] [PubMed] [Google Scholar]
- Lowe S. E., Theodorou M. K., Trinci A. P. Cellulases and xylanase of an anaerobic rumen fungus grown on wheat straw, wheat straw holocellulose, cellulose, and xylan. Appl Environ Microbiol. 1987 Jun;53(6):1216–1223. doi: 10.1128/aem.53.6.1216-1223.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison I. M. Changes in the hemicellulosic polysaccharides of rye-grass with increasing maturity. Carbohydr Res. 1974 Aug;36(1):45–51. doi: 10.1016/s0008-6215(00)81991-6. [DOI] [PubMed] [Google Scholar]
- Mountfort D. O., Asher R. A., Bauchop T. Fermentation of Cellulose to Methane and Carbon Dioxide by a Rumen Anaerobic Fungus in a Triculture with Methanobrevibacter sp. Strain RA1 and Methanosarcina barkeri. Appl Environ Microbiol. 1982 Jul;44(1):128–134. doi: 10.1128/aem.44.1.128-134.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountfort D. O., Asher R. A. Production and regulation of cellulase by two strains of the rumen anaerobic fungus Neocallimastix frontalis. Appl Environ Microbiol. 1985 May;49(5):1314–1322. doi: 10.1128/aem.49.5.1314-1322.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountfort D. O., Asher R. A. Production of alpha-Amylase by the Ruminal Anaerobic Fungus Neocallimastix frontalis. Appl Environ Microbiol. 1988 Sep;54(9):2293–2299. doi: 10.1128/aem.54.9.2293-2299.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountfort D. O., Asher R. A. Production of xylanase by the ruminal anaerobic fungus Neocallimastix frontalis. Appl Environ Microbiol. 1989 Apr;55(4):1016–1022. doi: 10.1128/aem.55.4.1016-1022.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountfort D. O., Asher R. A. Role of catabolite regulatory mechanisms in control of carbohydrate utilization by the rumen anaerobic fungus Neocallimastix frontalis. Appl Environ Microbiol. 1983 Dec;46(6):1331–1338. doi: 10.1128/aem.46.6.1331-1338.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orpin C. G. Studies on the rumen flagellate Sphaeromonas communis. J Gen Microbiol. 1976 Jun;94(2):270–280. doi: 10.1099/00221287-94-2-270. [DOI] [PubMed] [Google Scholar]
- Orpin C. G. The rumen flagellate Piromonas communis: its life-history and invasion of plant material in the rumen. J Gen Microbiol. 1977 Mar;99(1):107–117. doi: 10.1099/00221287-99-1-107. [DOI] [PubMed] [Google Scholar]
- Pearce P. D., Bauchop T. Glycosidases of the rumen anaerobic fungus Neocallimastix frontalis grown on cellulosic substrates. Appl Environ Microbiol. 1985 May;49(5):1265–1269. doi: 10.1128/aem.49.5.1265-1269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M. W., Gordon G. L. Sugar and polysaccharide fermentation by rumen anaerobic fungi from Australia, Britain and New Zealand. Biosystems. 1988;21(3-4):377–383. doi: 10.1016/0303-2647(88)90036-6. [DOI] [PubMed] [Google Scholar]
- Strobel H. J., Russell J. B. Regulation of beta-glucosidase in Bacteroides ruminicola by a different mechanism: growth rate-dependent derepression. Appl Environ Microbiol. 1987 Oct;53(10):2505–2510. doi: 10.1128/aem.53.10.2505-2510.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodorou M. K., Lowe S. E., Trinci A. P. The fermentative characteristics of anaerobic rumen fungi. Biosystems. 1988;21(3-4):371–376. doi: 10.1016/0303-2647(88)90035-4. [DOI] [PubMed] [Google Scholar]
- Weimer P. J., Zeikus J. G. Fermentation of cellulose and cellobiose by Clostridium thermocellum in the absence of Methanobacterium thermoautotrophicum. Appl Environ Microbiol. 1977 Feb;33(2):289–297. doi: 10.1128/aem.33.2.289-297.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. G., Orpin C. G. Glycoside hydrolase enzymes present in the zoospore and vegetative growth stages of the rumen fungi Neocallimastix patriciarum, Piromonas communis, and an unidentified isolate, grown on a range of carbohydrates. Can J Microbiol. 1987 May;33(5):427–434. doi: 10.1139/m87-072. [DOI] [PubMed] [Google Scholar]
- Williams A. G., Orpin C. G. Polysaccharide-degrading enzymes formed by three species of anaerobic rumen fungi grown on a range of carbohydrate substrates. Can J Microbiol. 1987 May;33(5):418–426. doi: 10.1139/m87-071. [DOI] [PubMed] [Google Scholar]
- Williams A. G. Rumen holotrich ciliate protozoa. Microbiol Rev. 1986 Mar;50(1):25–49. doi: 10.1128/mr.50.1.25-49.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolin M. J., Miller T. L. Interactions of microbial populations in cellulose fermentation. Fed Proc. 1983 Jan;42(1):109–113. [PubMed] [Google Scholar]


