Abstract
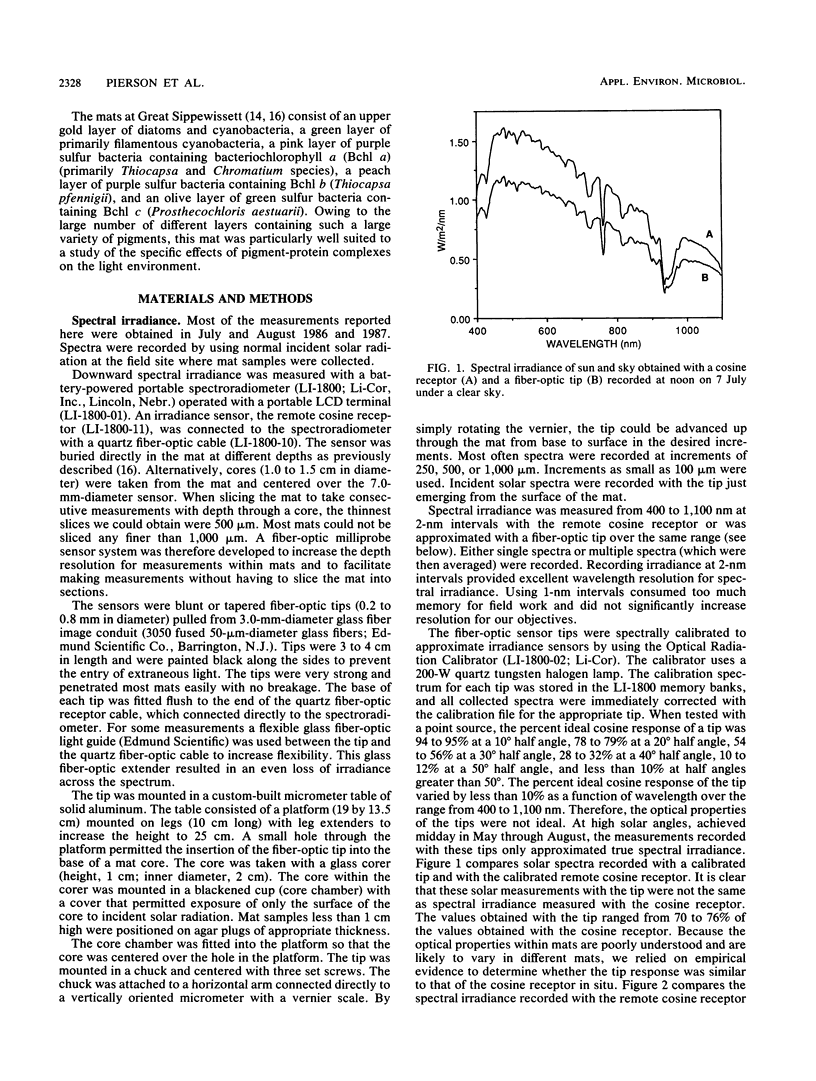
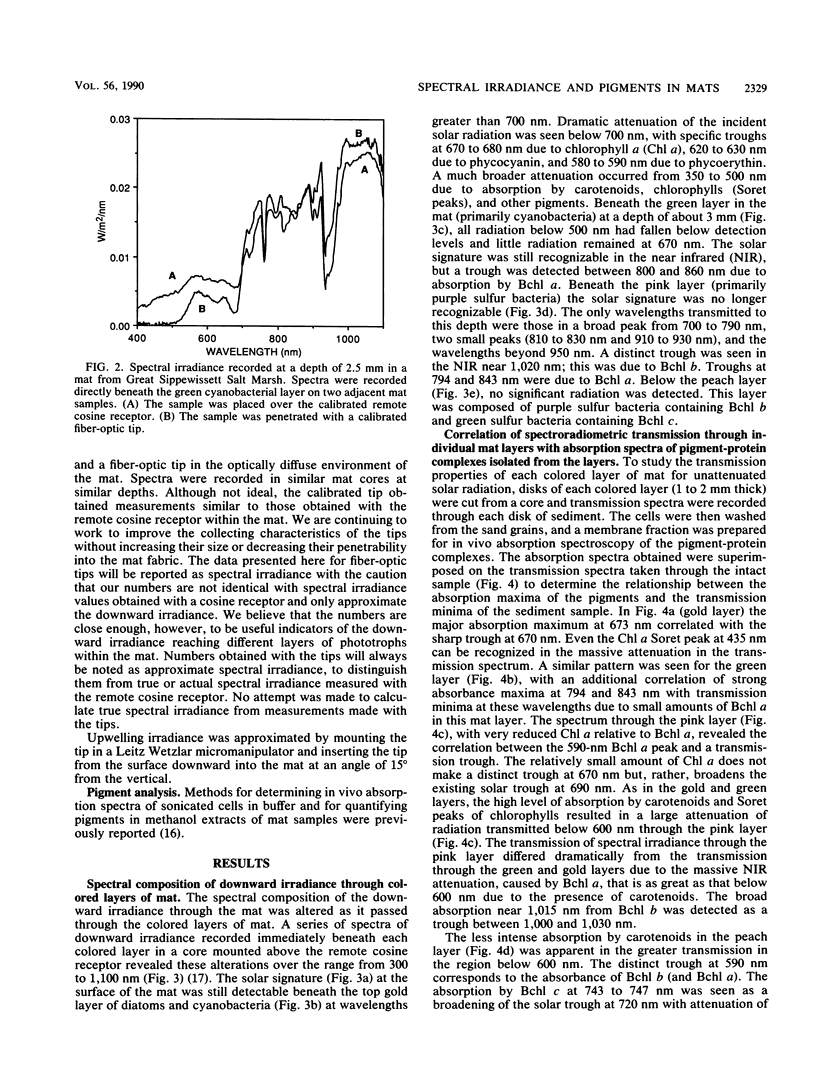
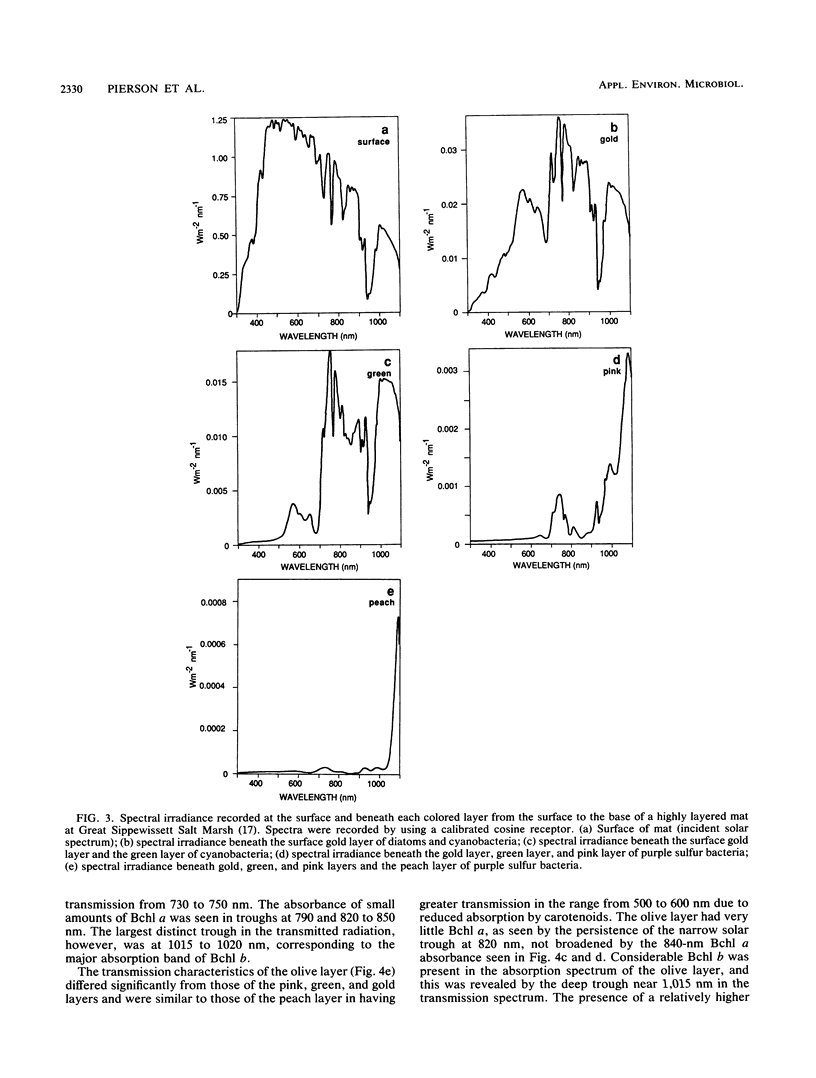
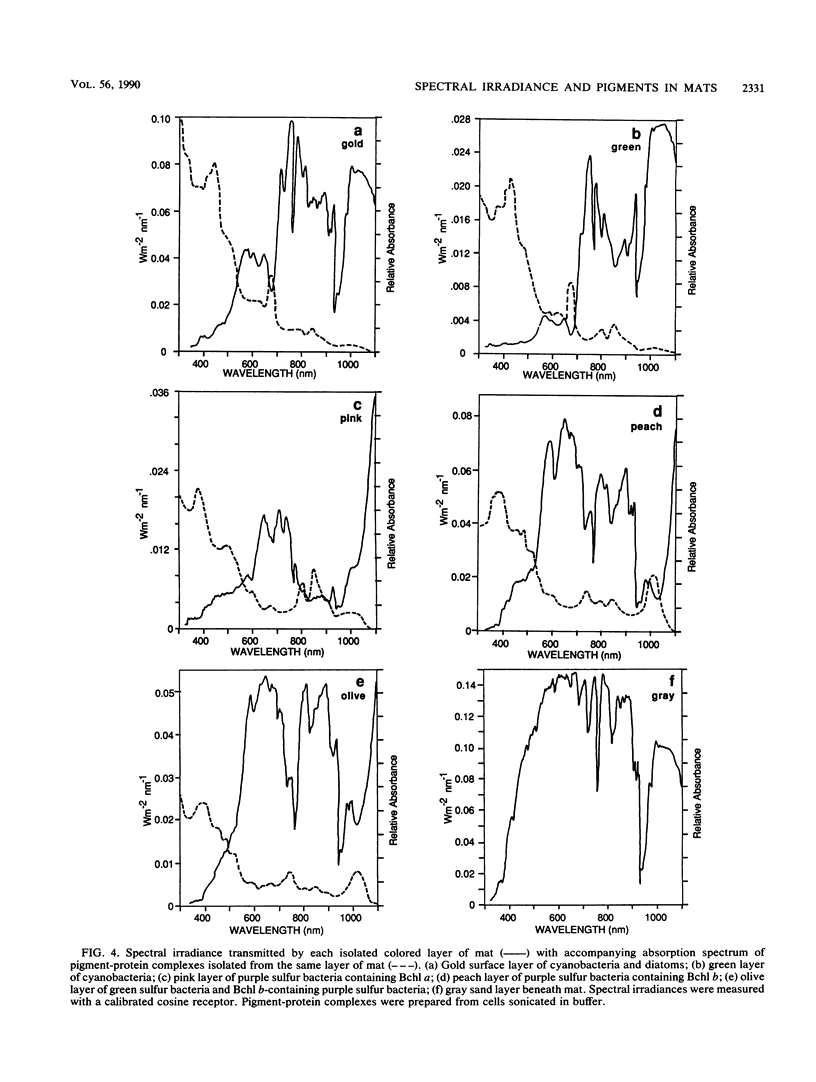
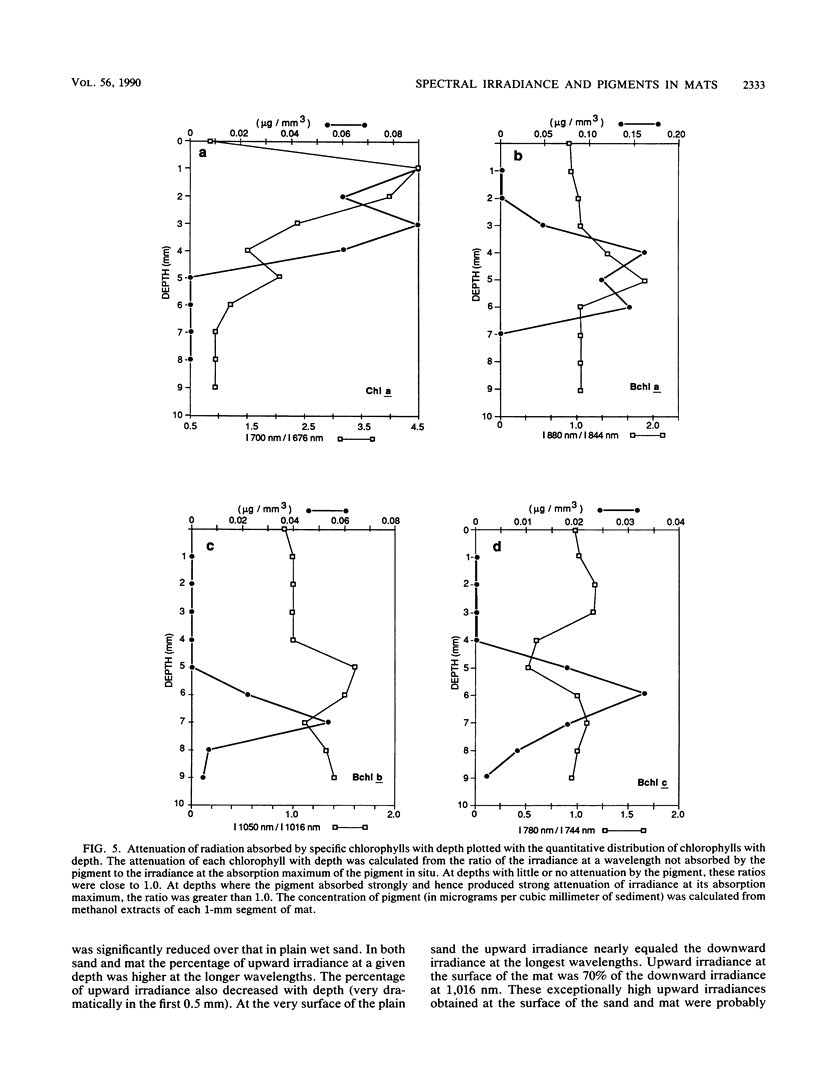
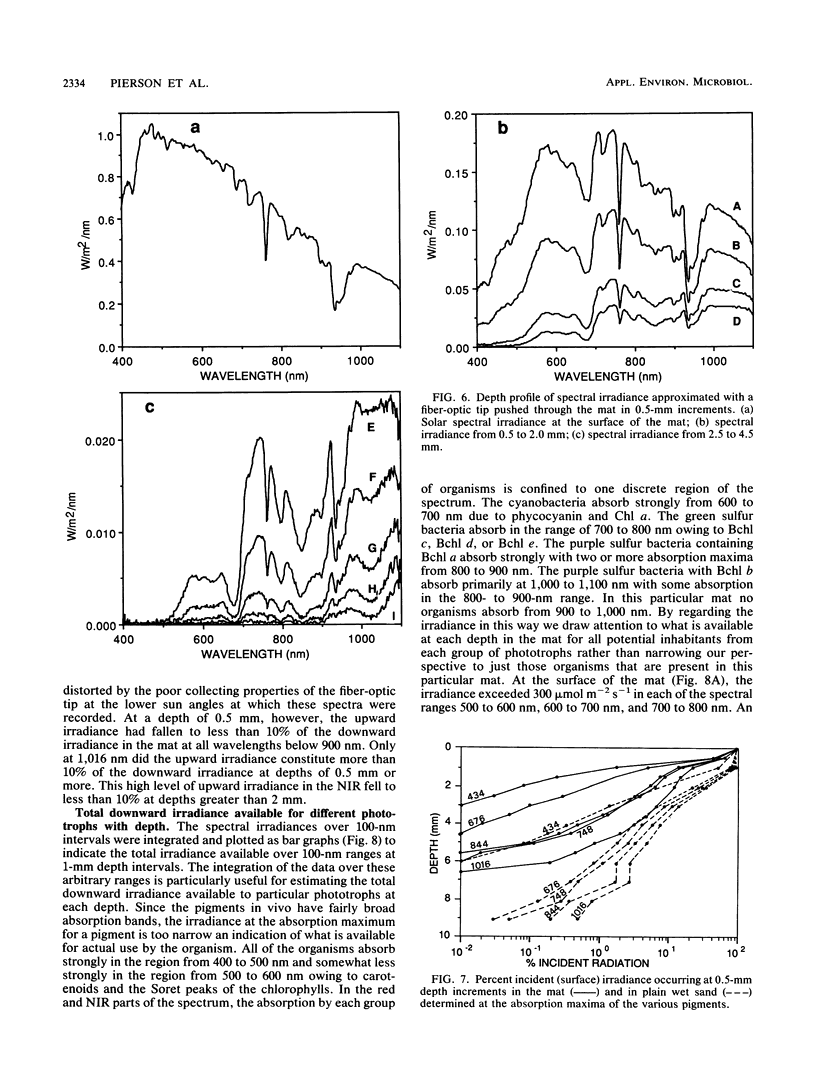
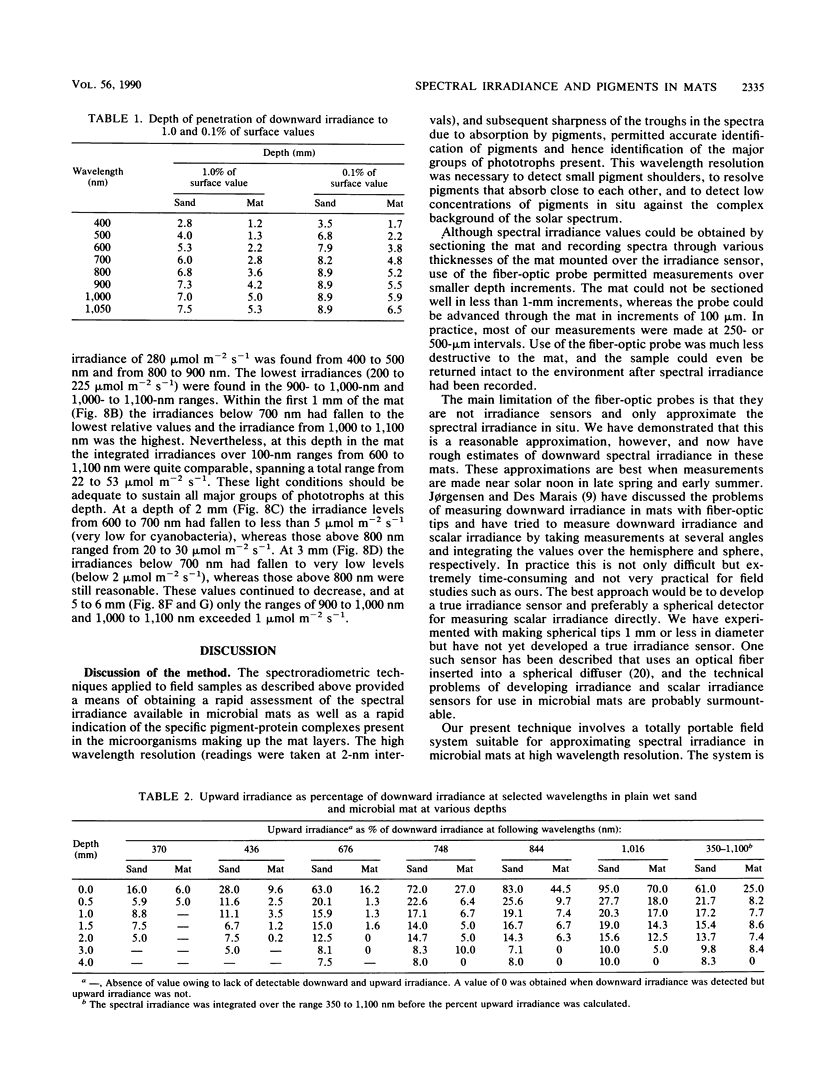
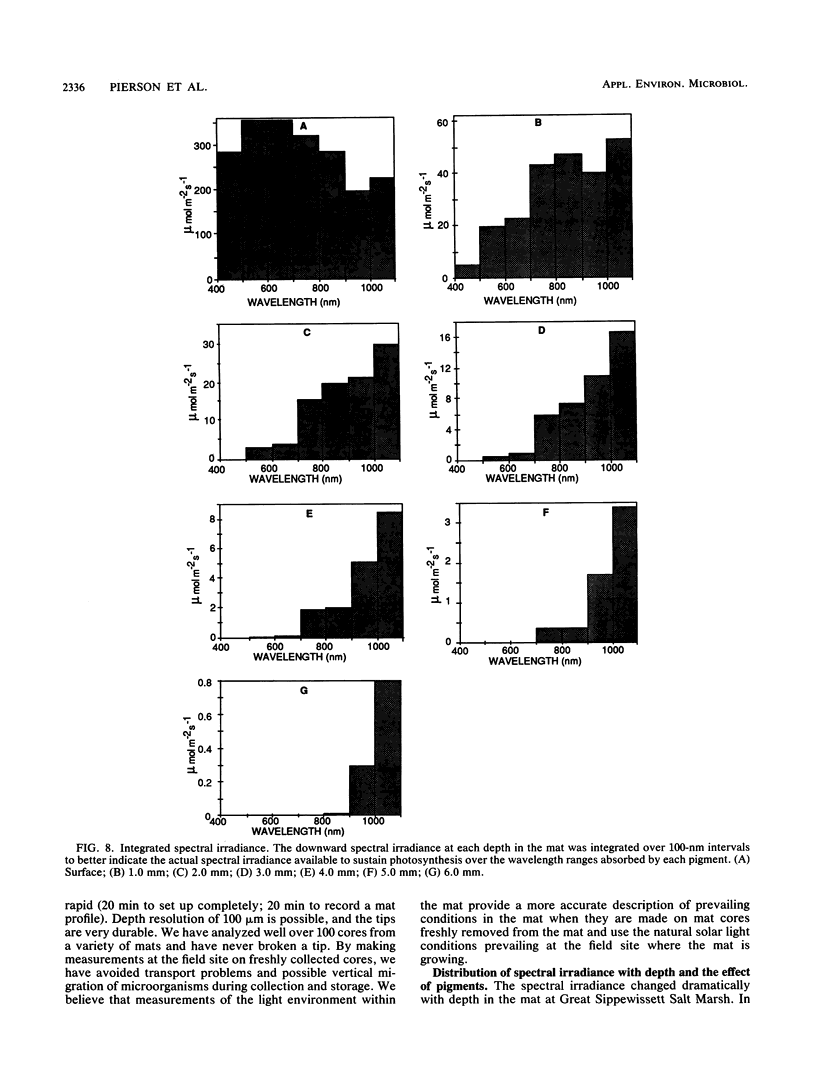
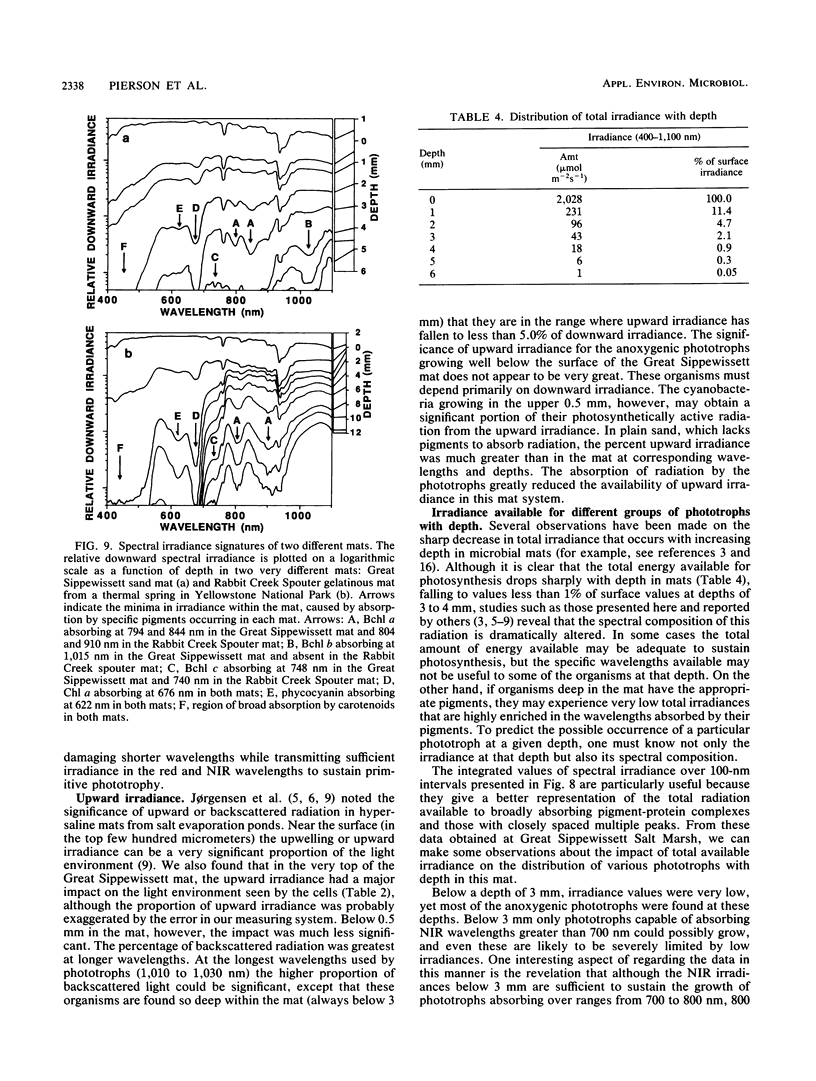
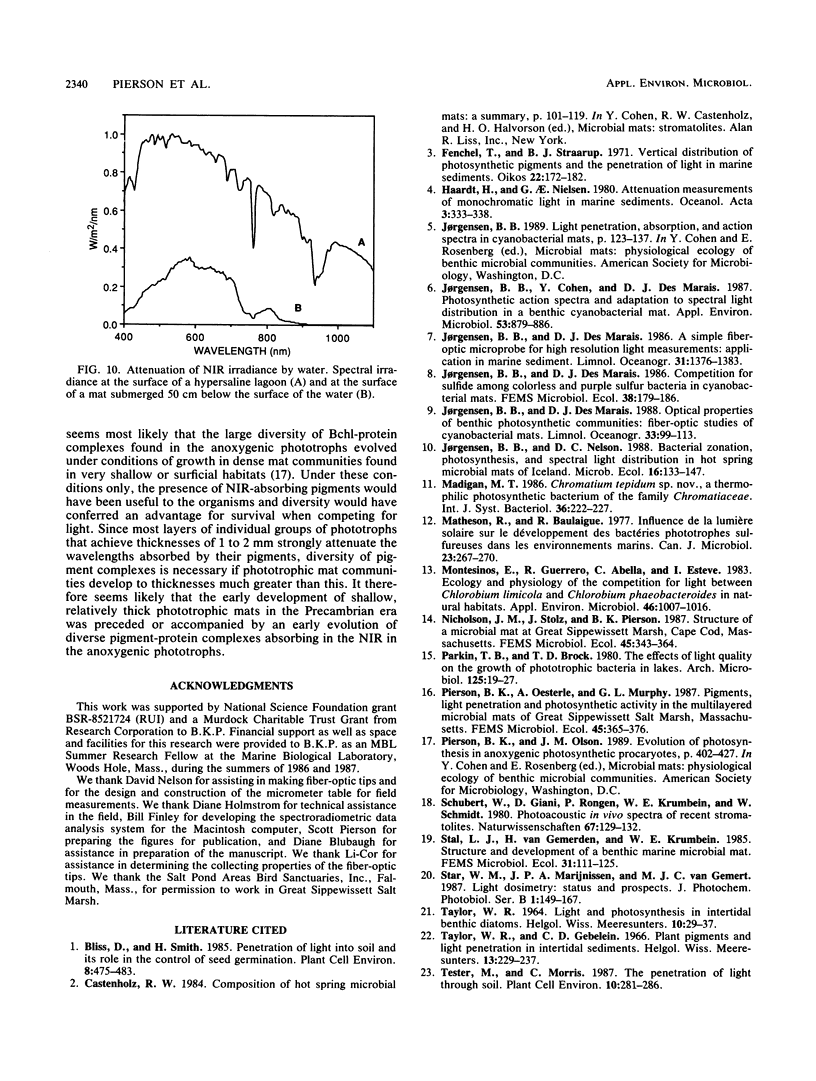
The spectral irradiance from 400 to 1,100 nm was measured with depth in the intertidal sand mats at Great Sippewissett Salt Marsh, Mass. These mats contained at least four distinct layers, composed of cyanobacteria, purple sulfur bacteria containing bacteriochlorophyll a (Bchl a), purple sulfur bacteria containing Bchl b, and green sulfur bacteria. Spectral irradiance was measured directly by layering sections of mat on a cosine receptor. Irradiance was also approximated by using a calibrated fiber-optic tip. With the tip, irradiance measurements could be obtained at depth intervals less than 250 μm. The irradiance spectra were correlated qualitatively and quantitatively with the distribution of the diverse chlorophyll pigments in this mat and were compared with spectra recorded in plain sand lacking pigmented phototrophs. We found that the shorter wavelengths (400 to 550 nm) were strongly attenuated in the top 2 mm of the mat. The longer wavelengths (red and near infrared) penetrated to much greater depths, where they were attenuated by Bchl a, b, and c-containing anoxygenic phototrophic bacteria. The specific attenuation bands in the irradiance spectra correlated with the specific in vivo absorption bands of the Bchl-protein complexes in the bacteria. We concluded that the pigments in the phototrophs had a profound affect on the light environment within the mat. It seems likely that the diverse Bchl-protein complexes found in the anoxygenic phototrophs evolved in dense mat environments as a result of competition for light.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Jorgensen B. B., Cohen Y., Des Marais D. J. Photosynthetic action spectra and adaptation to spectral light distribution in a benthic cyanobacterial mat. Appl Environ Microbiol. 1987 Apr;53(4):879–886. doi: 10.1128/aem.53.4.879-886.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen B. B., Des Marais D. J. A simple fiber-optic microprobe for high resolution light measurements: application in marine sediment. Limnol Oceanogr. 1986;31(6):1376–1383. doi: 10.4319/lo.1986.31.6.1376. [DOI] [PubMed] [Google Scholar]
- Jorgensen B. B., Des Marais D. J. Competition for sulfide among colorless and purple sulfur bacteria in cyanobacterial mats. FEMS Microbiol Ecol. 1986;38:179–186. doi: 10.1111/j.1574-6968.1986.tb01727.x. [DOI] [PubMed] [Google Scholar]
- Jorgensen B. B., Des Marais D. J. Optical properties of benthic photosynthetic communities: fiber-optic studies of cyanobacterial mats. Limnol Oceanogr. 1988;33(1):99–113. doi: 10.4319/lo.1988.33.1.0099. [DOI] [PubMed] [Google Scholar]
- Matheron R., Baulaigue R. Influence de la énétration de la lumière solaire sur le développement des bactéries phototrophes sulfureuses dans les environnements marins. Can J Microbiol. 1977 Mar;23(3):267–270. [PubMed] [Google Scholar]
- Montesinos E., Guerrero R., Abella C., Esteve I. Ecology and Physiology of the Competition for Light Between Chlorobium limicola and Chlorobium phaeobacteroides in Natural Habitats. Appl Environ Microbiol. 1983 Nov;46(5):1007–1016. doi: 10.1128/aem.46.5.1007-1016.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Star W. M., Marijnissen J. P., van Gemert M. J. Light dosimetry: status and prospects. J Photochem Photobiol B. 1987 Dec;1(2):149–167. doi: 10.1016/1011-1344(87)80023-4. [DOI] [PubMed] [Google Scholar]


