Abstract
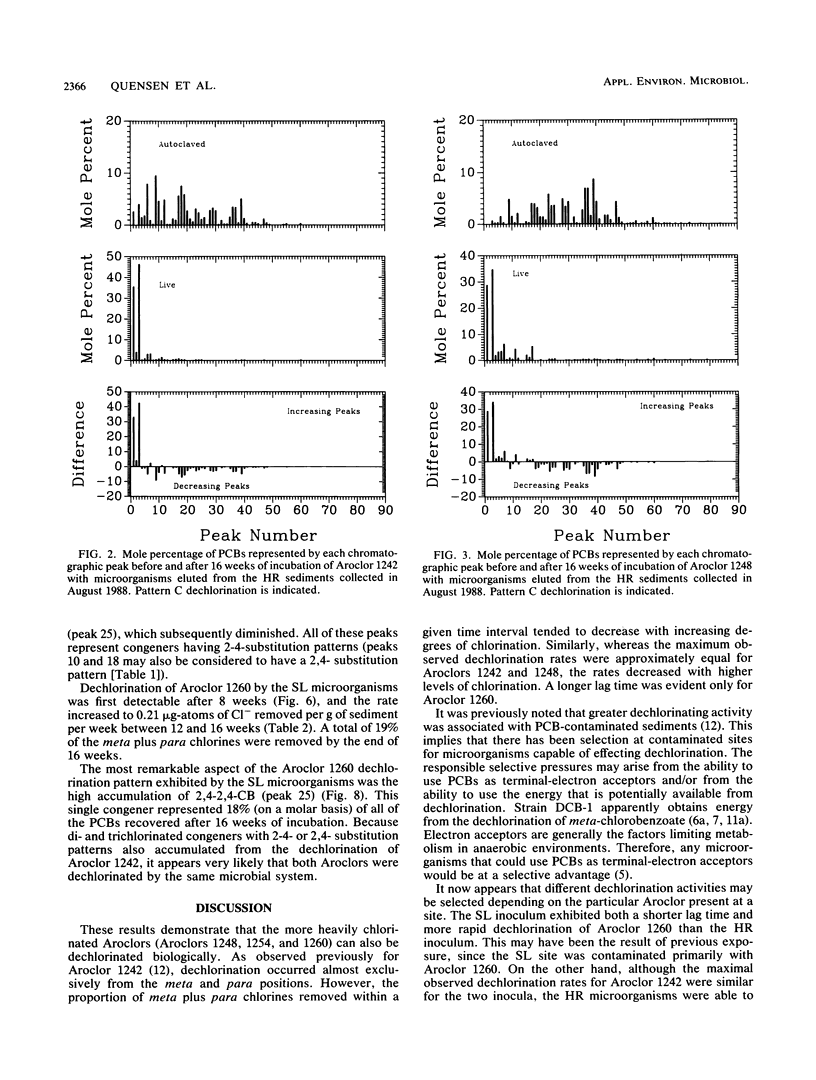
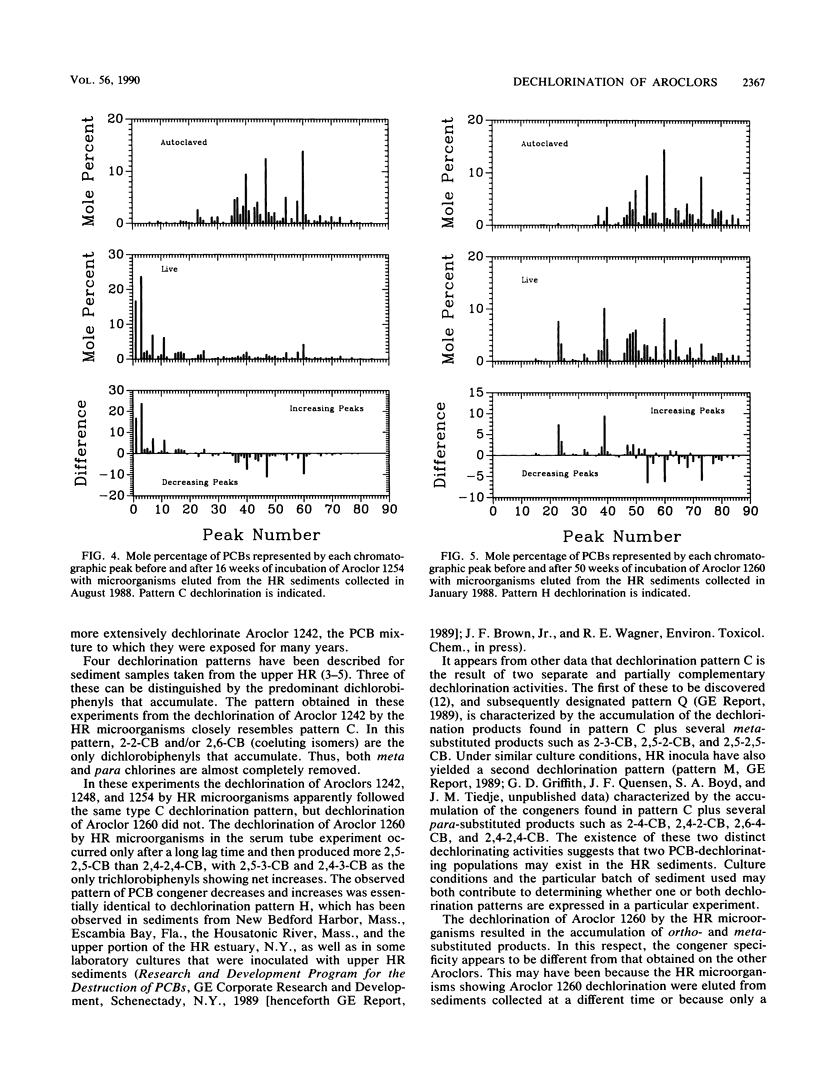
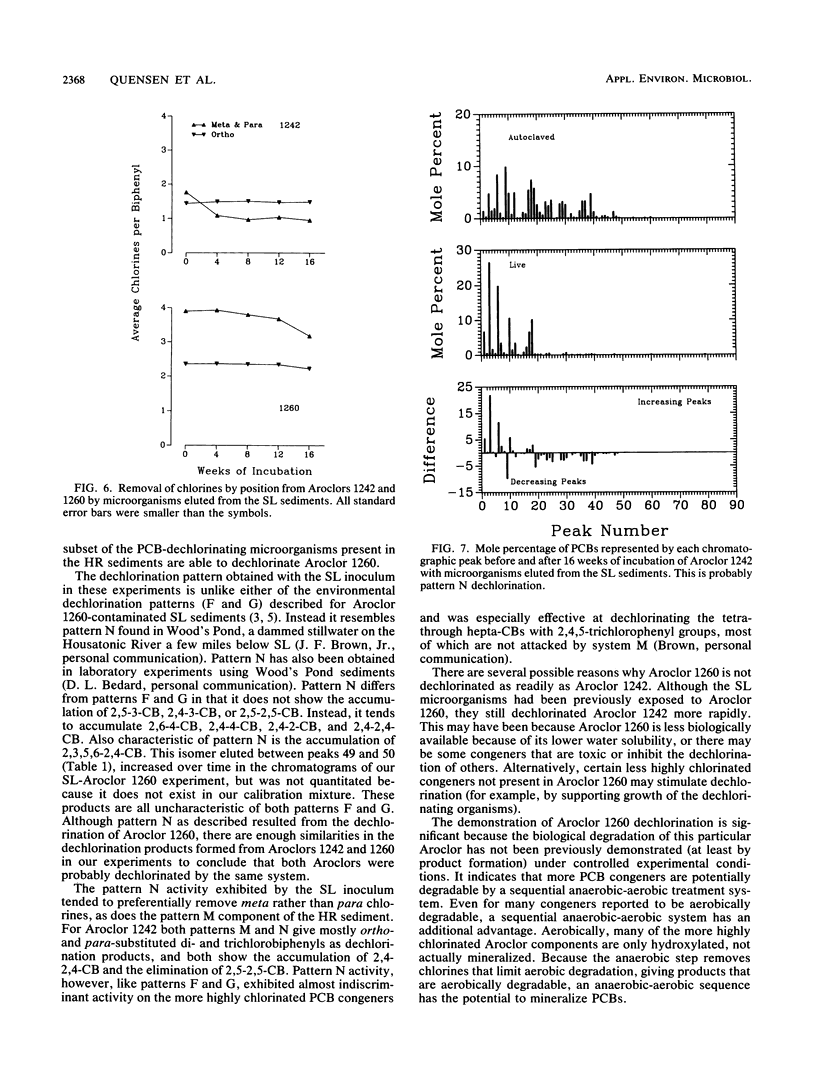
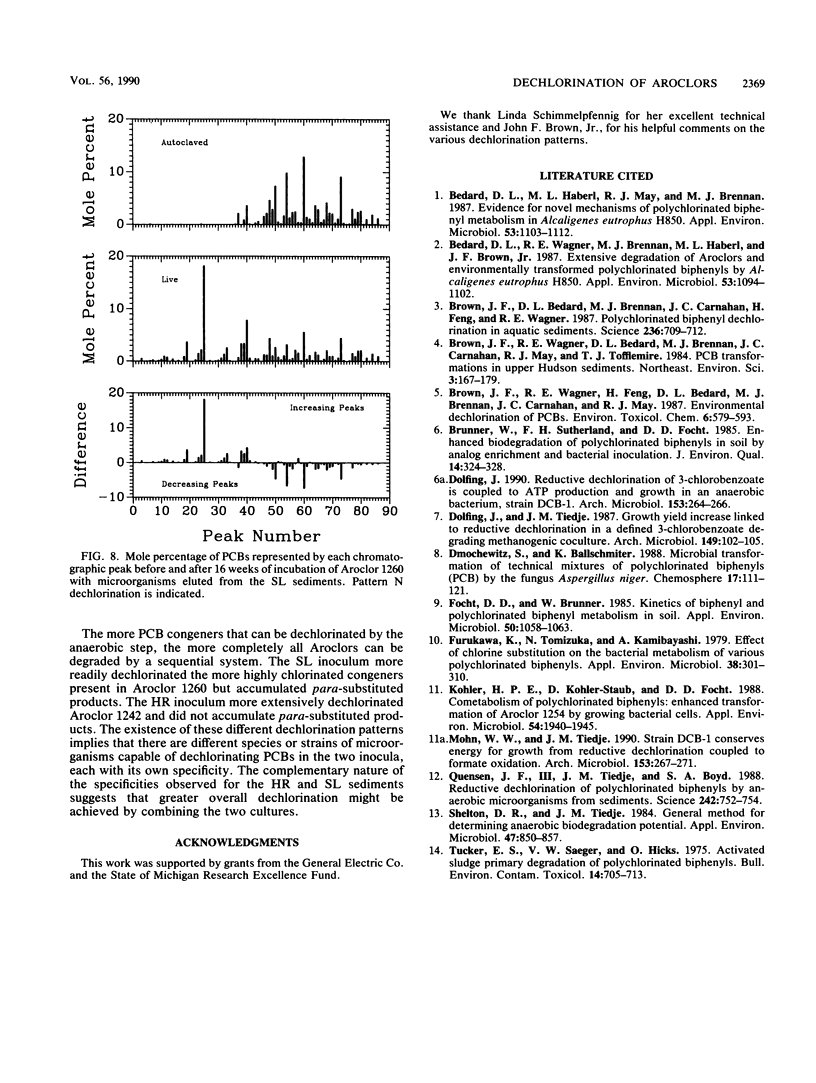
The rate, extent, and pattern of dechlorination of four Aroclors by inocula prepared from two polychlorinated biphenyl (PCB)-contaminated sediments were compared. The four mixtures used, Aroclors 1242, 1248, 1254, and 1260, average approximately three, four, five, and six chlorines, respectively, per biphenyl molecule. All four Aroclors were dechlorinated with the loss of meta plus para chlorines ranging from 15 to 85%. Microorganisms from an Aroclor 1242-contaminated site in the upper Hudson River dechlorinated Aroclor 1242 to a greater extent than did microorganisms from Aroclor 1260-contaminated sediments from Silver Lake, Mass. The Silver Lake inoculum dechlorinated Aroclor 1260 more rapidly than the Hudson River inoculum did and showed a preferential removal of meta chlorines. For each inoculum the rate and extent of dechlorination tended to decrease as the degree of chlorination of the Aroclor increased, especially for Aroclor 1260. The maximal observed dechlorination rates were 0.3, 0.3, and 0.2 μg-atoms of Cl removed per g of sediment per week for Aroclors 1242, 1248, and 1254, respectively. The maximal observed dechlorination rates for Hudson River and Silver Lake organisms for Aroclor 1260 were 0.04 and 0.21 μg-atoms of Cl removed per g of sediment per week, respectively. The dechlorination patterns obtained suggested that the Hudson River microorganisms were more capable than the Silver Lake organisms of removing the last para chlorine. These results suggest that there are different PCB-dechlorinating microorganisms at different sites, with characteristic specificities for PCB dechlorination.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedard D. L., Haberl M. L., May R. J., Brennan M. J. Evidence for novel mechanisms of polychlorinated biphenyl metabolism in Alcaligenes eutrophus H850. Appl Environ Microbiol. 1987 May;53(5):1103–1112. doi: 10.1128/aem.53.5.1103-1112.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard D. L., Wagner R. E., Brennan M. J., Haberl M. L., Brown J. F., Jr Extensive degradation of Aroclors and environmentally transformed polychlorinated biphenyls by Alcaligenes eutrophus H850. Appl Environ Microbiol. 1987 May;53(5):1094–1102. doi: 10.1128/aem.53.5.1094-1102.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. F., Jr, Bedard D. L., Brennan M. J., Carnahan J. C., Feng H., Wagner R. E. Polychlorinated biphenyl dechlorination in aquatic sediments. Science. 1987 May 8;236(4802):709–712. doi: 10.1126/science.236.4802.709. [DOI] [PubMed] [Google Scholar]
- Dolfing J. Reductive dechlorination of 3-chlorobenzoate is coupled to ATP production and growth in an anaerobic bacterium, strain DCB-1. Arch Microbiol. 1990;153(3):264–266. doi: 10.1007/BF00249079. [DOI] [PubMed] [Google Scholar]
- Dolfing J., Tiedje J. M. Growth yield increase linked to reductive dechlorination in a defined 3-chlorobenzoate degrading methanogenic coculture. Arch Microbiol. 1987;149(2):102–105. doi: 10.1007/BF00425073. [DOI] [PubMed] [Google Scholar]
- Focht D. D., Brunner W. Kinetics of biphenyl and polychlorinated biphenyl metabolism in soil. Appl Environ Microbiol. 1985 Oct;50(4):1058–1063. doi: 10.1128/aem.50.4.1058-1063.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K., Tomizuka N., Kamibayashi A. Effect of chlorine substitution on the bacterial metabolism of various polychlorinated biphenyls. Appl Environ Microbiol. 1979 Aug;38(2):301–310. doi: 10.1128/aem.38.2.301-310.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler H. P., Kohler-Staub D., Focht D. D. Cometabolism of polychlorinated biphenyls: enhanced transformation of Aroclor 1254 by growing bacterial cells. Appl Environ Microbiol. 1988 Aug;54(8):1940–1945. doi: 10.1128/aem.54.8.1940-1945.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohn W. W., Tiedje J. M. Strain DCB-1 conserves energy for growth from reductive dechlorination coupled to formate oxidation. Arch Microbiol. 1990;153(3):267–271. doi: 10.1007/BF00249080. [DOI] [PubMed] [Google Scholar]
- Quensen J. F., 3rd, Tiedje J. M., Boyd S. A. Reductive dechlorination of polychlorinated biphenyls by anaerobic microorganisms from sediments. Science. 1988 Nov 4;242(4879):752–754. doi: 10.1126/science.242.4879.752. [DOI] [PubMed] [Google Scholar]
- Shelton D. R., Tiedje J. M. General method for determining anaerobic biodegradation potential. Appl Environ Microbiol. 1984 Apr;47(4):850–857. doi: 10.1128/aem.47.4.850-857.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker E. S., Saeger V. W., Hicks O. Activated sludge primary biodegradation of polychlorinated biphenyls. Bull Environ Contam Toxicol. 1975 Dec;14(6):705–713. doi: 10.1007/BF01685246. [DOI] [PubMed] [Google Scholar]


