Abstract
Proper fatty acid metabolism is critical for hair and skin development and maintenance. The acyl-CoA binding protein (Acbp) is a widely expressed protein that binds long-chain fatty acyl-CoA esters and plays a role in fatty acyl-CoA transport and pool formation. However, loss of function of Acbp in the whole animal has not been investigated. Here, we show that deletion of Acbp in mouse results in sebocyte hyperplasia and sparse, matted hair with a greasy appearance. Consistent with these gross abnormalities, Acbp is highly expressed in the pilosebaceous units of mouse skin as determined by northern analysis and in situ hybridization. Loss of Acbp also results in fatty acid metabolism abnormalities, with hair lipid profiles showing altered levels of triacylglycerols and nearly co-migrating lipids. These data suggest that Acbp plays a role in triacylglycerol biosynthesis and that regulation of this process is important for proper hair and skin development and maintenance in the mouse.
INTRODUCTION
Long-chain fatty acyl-CoA esters function as substrates and intermediates in lipid biosynthesis and catabolism and also play a role in regulating carbohydrate metabolism, protein sorting, gene expression, and signal transduction (Faergeman and Knudsen, 1997; Knudsen et al., 2000). Homeostatic control of these molecules is therefore essential for numerous cellular functions.
The acyl-CoA binding protein (Acbp), which was also identified as the diazepam binding inhibitor (Mocchetti and Santi, 1991), is a 10 kD, cytoplasmic protein that binds medium- and long-chain fatty acyl-CoA esters and plays a role in fatty acid metabolism (Knudsen et al., 1999). Acbp is found in eukaryotes ranging from yeast to mammals (Knudsen et al., 1999). Although testis- and brain-specific isoforms have been identified, most tissues express a common form (Knudsen et al., 1999).
Several studies have indicated that Acbp contributes to intracellular acyl-CoA transport and pool formation. In vitro, Acbp binds C14-C22 acyl-CoA esters with high affinity but does not bind free fatty acids, acyl carnitines, or cholesterol (Rasmussen et al., 1990; Rosendal et al., 1993). Acbp protects long-chain acyl-CoA esters from hydrolysis by microsomal hydrolases and can donate acyl-CoA for mitochondrial oxidation and microsomal glycerolipid synthesis (Rasmussen et al., 1994; Rasmussen et al., 1993). Specifically, in vitro, binding of long-chain fatty acyl-CoAs to Acbp inhibits their incorporation into triacylglycerols in microsomal membranes, whereas incorporation into phospholipids is relatively unaffected, suggesting that Acbp preferentially donates fatty acyl-CoAs for neutral glycerolipid synthesis (Rasmussen et al., 1993). Over-expression of the bovine or Saccharomyces cerevisiae orthologs in yeast results in an increase in the intracellular acyl-CoA ester pool size (Mandrup et al., 1993; Schjerling et al., 1996). Conversely, disruption of the yeast ortholog results in an accumulation of long-chain acyl-CoA esters, particularly octadecanoyl-CoA (C18:0), with no change in fatty acyl-CoA synthesis (Schjerling et al., 1996). Addition of Acbp causes a dramatic decrease in the average chain length of acyl-CoA esters, suggesting that Acbp may function in transport of acyl-CoA esters from the site of synthesis to the intracellular pools where they are available for enzymes involved in their processing and catabolism. Overall, these studies indicate that Acbp plays a role in the availability of long-chain fatty acyl-CoA esters for a variety of biochemical processes.
To date, most Acbp functional studies have been performed in vitro or in yeast. Reduction of Acbp in a variety of human cell lines by small interference RNA results in cell lethality, suggesting that Acbp performs an essential function (Faergeman and Knudsen, 2002). Recently, transgenic over-expression of Acbp in mice was shown to result in an increase in liver long-chain fatty acyl-CoA pool size, particularly levels of saturated and polyunsaturated fatty acyl-CoAs (Huang et al., 2005). However, loss of function has not been investigated in vivo.
In this paper, we describe hair and skin abnormalities in mice homozygous for the nm1054 mutation, which was previously described as a recessive, pleiotropic mutation caused by an approximately 400 kb deletion on chromosome 1 (Ohgami et al., 2005a; Ohgami et al., 2005b). Here, we characterize hair and skin abnormalities in nm1054 deletion mutants and show that these phenotypes result from a loss of Acbp and are related to a defect in cutaneous fatty acid metabolism.
RESULTS
Cutaneous abnormalities in nm1054 mice
As they develop their adult coat, B6129F1 mice homozygous for the nm1054 deletion have sparse, reddish, matted hair with a greasy appearance (Figure 1a–c). To determine the nature of the cutaneous abnormality, we examined the histological appearance of adult skin from a variety of locations. The epidermis and dermis appear normal in nm1054 mutants, and there is a normal distribution of pilosebaceous units (PSUs), which are comprised of hair follicles and sebocytes. However, consistent with the greasy appearance of nm1054 hair, there is an increased number of sebocytes associated with most PSUs from skin of the lower thorax (Figure 2a–b) and the nose (Figure 2c–d) of nm1054 deletion homozygotes. No differences were observed in the skin from the paws (Figure 2e–f) or the tail (Figure 2g–h), or in juvenile skin (data not shown).
Figure 1. The nm1054 hair phenotype.
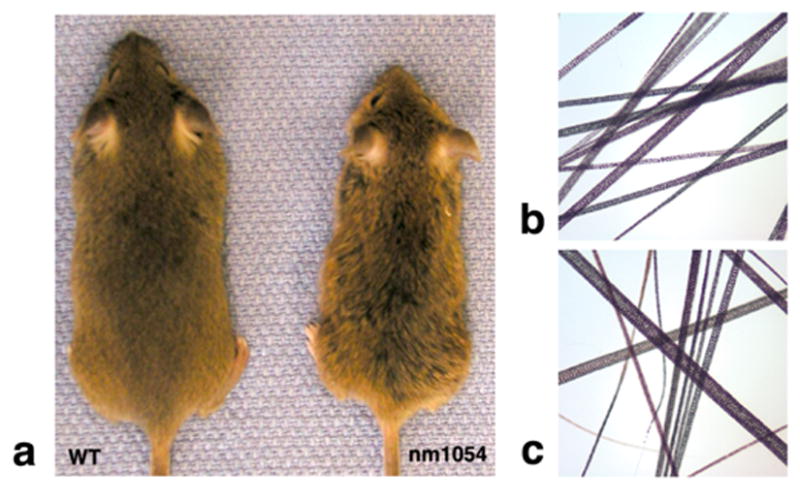
(a) Appearance of eight week, male, B6129F1 wild type (left) and nm1054 deletion homozygous (right) mice. Note the matted, greasy appearance of the nm1054 fur. (b) Hair from the back of an eight week, male, B6129F1 nm1054 heterozygote. Magnification is 10X. (c) Hair from the back of an eight week, male, B6129F1 nm1054 deletion homozygote. The mutant hair appears more reddish in color. Magnification is 10X.
Figure 2. Histological analysis of skin from eight week, male, wild type and nm1054 mice.
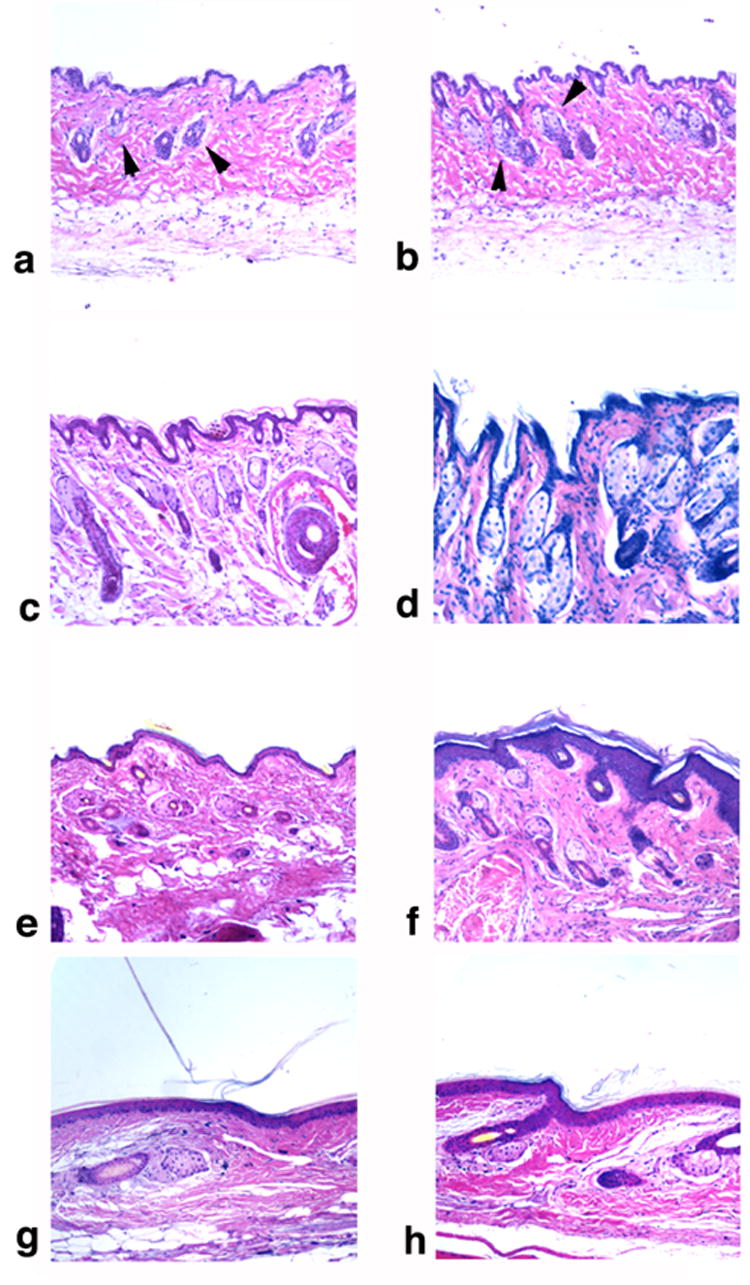
(a–b) Sections of wild type (a) and nm1054 (b) skin from the lower thorax. (c–d) Sections of wild type (c) and nm1054 (d) skin from the nose. (e–f) Sections of wild type (e) and nm1054 (f) skin from the paws. (g–h) Sections of wild type (g) and nm1054 (h) skin from the tail. Arrowheads indicate pilosebaceous units (PSUs). Magnification is 10X. All sections are stained with hematoxylin and eosin.
Transgenic rescue of the cutaneous phenotype
A BAC contig of the nm1054 deletion interval was developed previously (Ohgami et al., 2005b). Two transgenic lines derived from CITB BAC clone 486B9 (Kim et al., 1996) rescue the gross and microscopic cutaneous phenotypes but not the other nm1054 phenotypes (Figure 3a–c; (Ohgami et al., 2005a). Based on sequence analysis, two genes are predicted to be present on BAC 486B9: Acbp and a novel gene, which will be referred to by its GenBank accession number BAB29121 (Carninci and Hayashizaki, 1999). The appearance of the hair and the sebocyte hyperplasia in deletion mutants, combined with the documented role of Acbp in fatty acid metabolism, suggested that Acbp was a strong candidate for the nm1054 cutaneous phenotype.
Figure 3. BAC transgenic rescue of the nm1054 cutaneous abnormality.
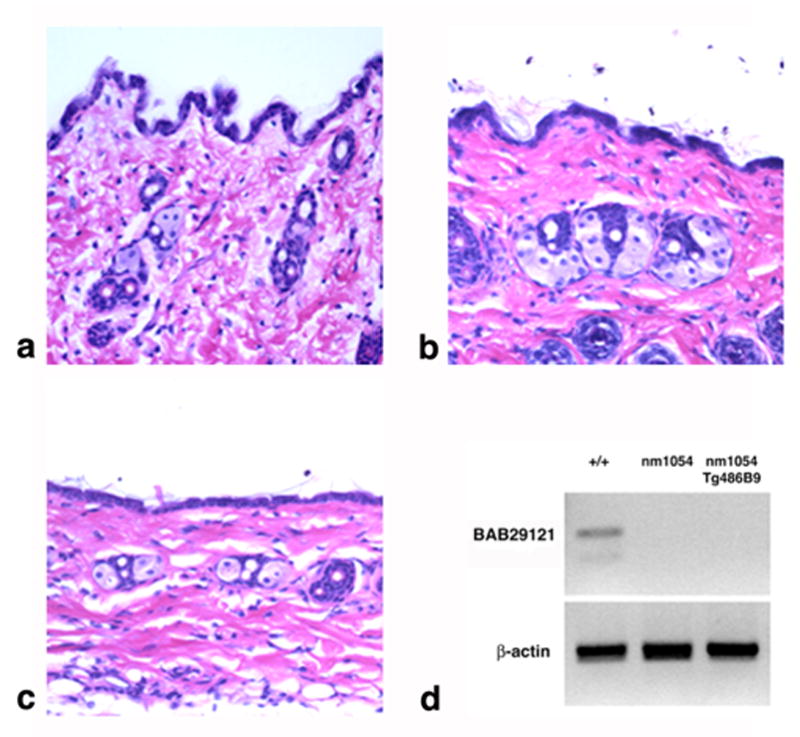
(a–c) Histology of wild type (a), nm1054 (b), and BAC 486B9 transgenic nm1054 (c) skin from the lower thorax. Magnification is 20X. All sections are stained with hematoxylin and eosin. (d) RT PCR of BAB29121 from mouse thymus RNA. β-actin was used as a loading control.
The BAB29121 gene has five exons and encodes a novel, 121 amino acid predicted protein with no identifiable domains and no homology to any known proteins. ESTs have been sequenced from thymus, uterus, mammary gland, lymph node, and brain, and there are predicted orthologs in species ranging from yeast to humans. BAB29121 is expressed in wild type mouse thymus but not in nm1054 or BAC 486B9 transgenic nm1054 animals (Figure 3d), suggesting that the gene or regulatory elements may extend beyond the limits of the BAC or are disrupted by insertion of circularized BAC DNA. Alternatively, the gene could be silenced due to a positional insertion effect. Lack of BAB29121 expression in BAC 486B9 transgenic nm1054 animals further suggests that the cutaneous phenotypes result solely from a loss of Acbp.
Expression of Acbp
Acbp expression was previously documented in murine brain, liver and kidney by northern analysis (Owens et al., 1989). Expression of the rat and bovine orthologs has also been reported in many tissues (Bovolin et al., 1990; Mikkelsen and Knudsen, 1987; Mocchetti et al., 1986). A murine northern blot shows strong Acbp expression in most tissues, with the highest expression in liver and skin and lower levels in spleen, muscle, and thymus (Figure 4a).
Figure 4. Expression of mouse Acbp.
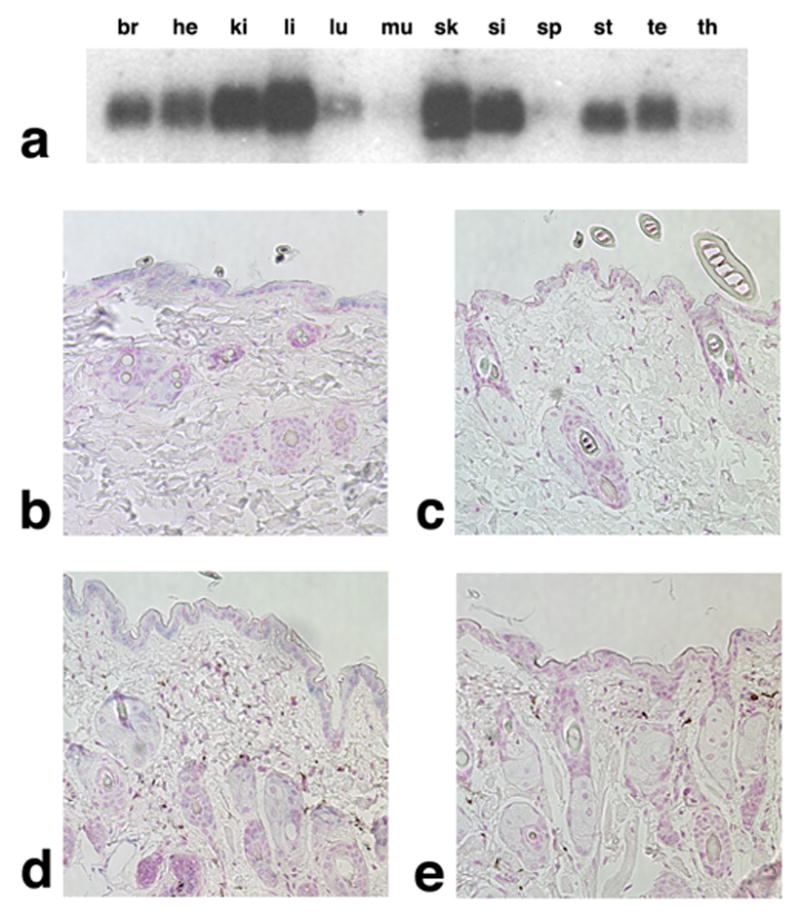
(a) Northern analysis of Acbp mRNA expression in brain (br), heart (he), kidney (ki), liver (li), lung (lu), muscle (mu), skin (sk), small intestine (si), spleen (sp), stomach (st), testis (te), and thymus (th). (b–c) In situ hybridization analysis of Acbp expression in eight week, male, wild type (b) and nm1054 (c) mouse skin from the lower thorax. (d–e) In situ hybridization analysis of Acbp expression in eight week, male, wild type (d) and nm1054 (e) mouse skin from the nose. Blue staining indicates Acbp expression in the epidermis, hair follicles, and sebocytes. Magnification is 20X.
Because of the abnormalities in nm1054 skin and the high level of Acbp expression in skin by northern analysis, we performed in situ hybridization for Acbp mRNA. Acbp expression was detected in the epidermis, hair follicles, and sebocytes of skin from the lower thorax (Figure 4b). This finding is consistent with ACBP protein expression in the epidermis and sebaceous glands of human skin (Alho et al., 1993). Similar epidermal and PSU expression was detected in skin from the nose (Figure 4d). No expression was detected in nm1054 skin (Figure 4c,e). Taken together with the function of Acbp in lipid metabolism and the absence of BAB29121 expression in BAC transgenic nm1054 mutants, these data provide further evidence that Acbp is responsible for the nm1054 hair and skin abnormalities.
Lipid analysis in nm1054 mice
Since in vitro studies had previously implicated Acbp in binding and metabolism of acyl-CoA esters, as well as facilitating production of various acylated lipids, we carried out global lipid analyses in several tissues from wild type, nm1054, and BAC 486B9 transgenic nm1054 mice. We used multiple methods to develop complete profiles for lipids from liver, skin, and hair. Although we detected no differences in the profiles of liver or skin lipids (Figure S1, Figure S2, and data not shown), distinctive differences were observed in the profile of lipids extracted from hair.
Silica thin layer chromatography (TLC) and high performance liquid chromatography-mass spectrometry (HPLC-MS) demonstrated no significant differences in the levels of most major classes of polar and non-polar hair lipids from wild type, nm1054, and BAC 486B9 transgenic nm1054 mice (Figure 5a, Figure S1, Figure S2, data not shown). However, we reproducibly observed a band unique to nm1054 hair and a species present in all three genotypes that was reduced in relative intensity in the nm1054 mutant (Figure 5a). Based on the similar migration of the common and unique bands, we inferred that these were likely structurally related lipids and attempted to identify both using an extensive battery of methods. The band unique to nm1054 did not give strong ions in mass spectrometry, and we were unable to obtain sufficient amounts to obtain a coherent nuclear magnetic resonance spectrum (data not shown). Consequently, we were unable to identify its structure. However, commensurate with its co-migration with the triacylglycerol standard tristearin (Figure 5a), positive mode mass spectrometry in the presence of lithium revealed that the common band represents triacylglycerols, with the strongest ion of m/z 865.7 corresponding to the expected mass of a lithium adduct of a triacylglycerol with a total fatty acyl chain length of C52 and two unsaturations (Figure 5b). Collision-induced mass spectrometry identified ions corresponding to products derived from the loss of C18:1 and C16:0 fatty acyl chains, confirming the identity of this lipid (Figure 5c). Overall, these data indicate that nm1054 mice produce sebaceous lipids with reduced levels of triacylglycerols accompanied by the appearance of an unidentified, nearly co-migrating lipid.
Figure 5. Chromatographic analysis of hair lipids.
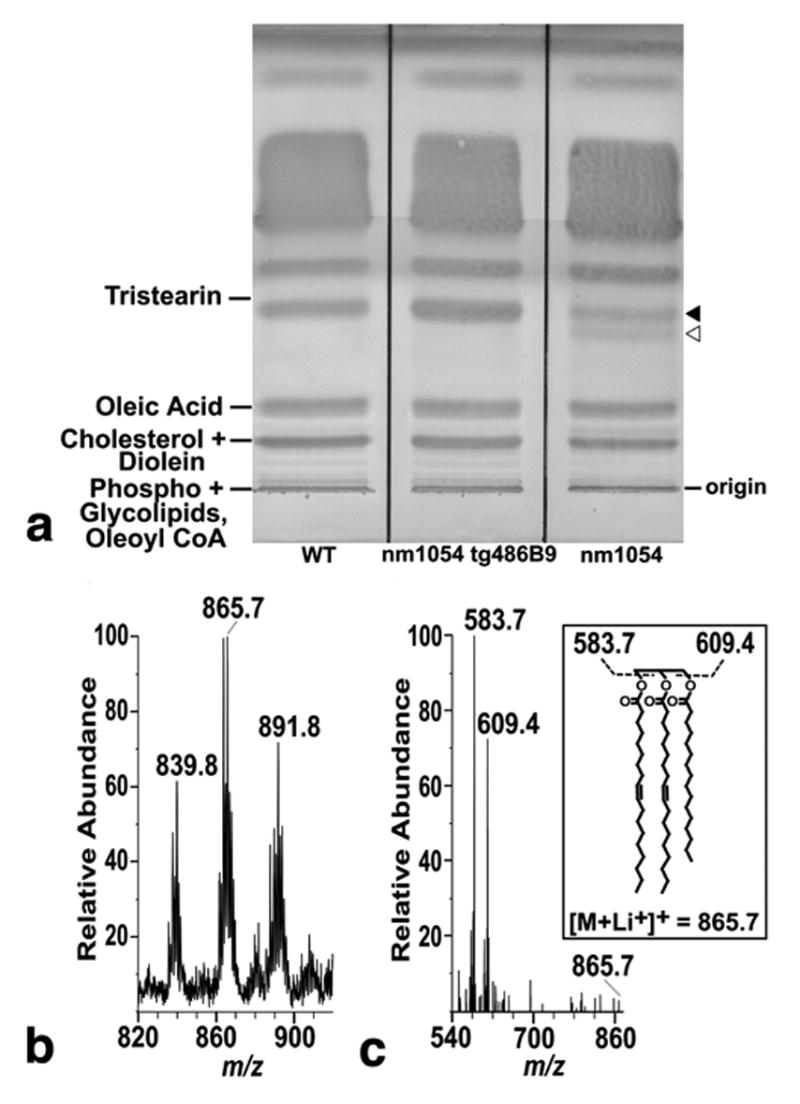
(a) Preparative silica TLC of lipids extracted from wild type, BAC 486B9 transgenic nm1054, and nm1054 hair showing a lower intensity band (closed arrowhead) in the nm1054 hair nearly co-migrating with a triacylglycerol standard (tristearin), as well as a unique band (open arrowhead) not present in wild type or transgenic mutant hair. (b) Positive mode mass spectrometric analysis with lithium of nm1054 hair lipids indicated by the closed arrow in Figure 5a, yielding ions of m/z 839.8, 865.7, and 891.8. The ion at m/z 865.7 corresponds to the expected mass of a lithium adduct of a triacylglycerol. (c) Collision-induced mass spectrometry spectrum of the ion at m/z 865.7 shows product ions corresponding to lithium adducts of diacylglycerol units after the loss of C18:1 (m/z 583.7) and C16:0 (m/z 609.4), confirming the initial structure of a triacylglycerol with two C18:1 and one C16:0 fatty acyl units (inset). The positions of fatty acids and unsaturations are shown arbitrarily.
DISCUSSION
In this study, we have uncovered an important role for the acyl-CoA binding protein in mouse hair and skin. We have demonstrated that the gene encoding the acyl-CoA binding protein is expressed at high levels in the pilosebaceous units of mouse skin and that loss of Acbp is responsible for the abnormal cutaneous phenotype of nm1054 mice. Consistent with the reported role of Acbp in lipid metabolism and its preferential role in synthesis of neutral glycerolipids, we identified a selective abnormality in triacylglycerols present on nm1054 hair.
Acbp joins a growing list of fatty acid metabolism genes in which mutations result in selective or predominant cutaneous phenotypes. Among these proteins is fatty acid transport protein 4 (Fatp4), which functions as an acyl-CoA synthetase and plays an important role in uptake and metabolism of long and very long chain fatty acids (Hall et al., 2005; Hirsch et al., 1998; Stahl et al., 1999). Loss of Fatp4 results in neonatal lethality due to restrictive dermopathy, a condition characterized by abnormally thin and tight skin with an expanded epidermis, a restricted dermis, and a reduced number of PSUs (Herrmann et al., 2003; Moulson et al., 2003). Similarly, Elovl3 belongs to a family of enzymes involved in the biosynthesis of very long chain fatty acids (Tvrdik et al., 2000) and is expressed primarily in the sebaceous glands and hair follicles of the skin (Westerberg et al., 2004). Mice deficient for Elovl3 have sparse fur, an expanded epidermis, and enlarged sebocytes (Westerberg et al., 2004). Loss of the gene encoding the acyl-CoA:diacylglycerol acyltransferase, a key enzyme in diacylglycerol synthesis, results in sparse, dry hair and a decreased number of sebocytes (Chen et al., 2002). Mice lacking stearoyl-CoA desaturase, which is involved in monounsaturated fatty acid biosynthesis, have atrophic sebocytes and a decreased level of triglycerides and cholesterol esters in the skin (Miyazaki et al., 2001). Finally, sparse hair and cyst-like inclusions in the skin of scraggly mice result from an abnormal lipid distribution in the hair and skin (Herron et al., 1999). These studies, along with our own, indicate that proper fatty acid metabolism is critical for normal hair and skin development and maintenance.
Acbp is thought to bind medium- and long-chain fatty acyl-CoA esters and donate them for use in other processes, including triacylglycerol biosynthesis (Rasmussen et al., 1994; Rasmussen et al., 1993). TLC analysis shows that nm1054 hair has a relative decrease in triacylglycerols and an accompanying increase in a novel, closely migrating band that may represent a related lipid, suggesting a defect in triacylglycerol metabolism. Despite extensive characterization, we were unable to identify the novel lipid under conditions where common lipids were readily detected (Figure 5b; data not shown).
The increase in sebocytes and the altered lipid profile undoubtedly contribute to the matted, greasy appearance of the adult coat. It is possible that the sebocyte hyperplasia itself is due to an increase in peroxisome proliferator-activated receptor (PPAR)-mediated gene transcription. The PPARs are nuclear hormone receptors that are activated by binding to fatty acids (Forman et al., 1997; Kliewer et al., 1997; Krey et al., 1997) and regulate transcription of a plethora of genes involved in lipid metabolism (Gervois et al., 2000; Kota et al., 2005; Smith, 2002) and skin development (Di-Poi et al., 2004; Rosenfield et al., 2000). Loss of the gene encoding the PPARγsubtype is embryonic lethal (Rosen et al., 1999). However, analysis of chimeric PPARγ null mice showed that PPARγ is required for sebocyte differentiation. Furthermore, expression of PPARγ in cultured cells promotes sebocyte maturation, lipid accumulation, and triacylglycerol synthesis (Rosenfield et al., 1999; Schadinger et al., 2005).
Consistent with the role of PPARγ in sebocyte differentiation, PPARγ and Acbp may function interdependently in fatty acid metabolism. Acbp and PPARγ are both expressed in the epidermis, hair follicles, and sebocytes of mouse skin (Figure 4; (Di-Poi et al., 2004; Rosenfield et al., 2000), and these cell lineages share a common origin and can affect differentiation and function of one another (Ferraris et al., 1997; Oshima et al., 2001; Reynolds and Jahoda, 1992; Taylor et al., 2000). Acbp is up-regulated by PPARγ and is thought to compete for binding to fatty acids that stimulate PPARγ-dependent transcription (Helledie et al., 2000; Helledie et al., 2002a). The processes of fatty acid conversion to CoA esters and reversion back to free fatty acids are in constant equilibrium, with CoA esters preferentially binding Acbp and free fatty acids binding PPARγ (Helledie et al., 2002b; Hertz et al., 1994; Hunt et al., 1999). An increase in Acbp expression or an increase in the level of fatty acyl-CoA esters decreases PPARγ-mediated transcription, presumably by reducing the level of free fatty acids available for binding to PPARγ (Helledie et al., 2000; Helledie et al., 2002a). Therefore, in the absence of Acbp, fatty acids may be in excess and available to stimulate PPARγ-mediated transcription, which could eventuate into sebocyte hyperplasia and alterations in hair lipid composition. Alternatively, it is entirely possible that changes in triacylglycerol metabolism may regulate transcription of other signals in a PPAR-independent manner.
Although Acbp is expressed in all tissues, we did not detect morphological or lipid abnormalities in any tissue other than hair or skin. To this end, there may be proteins functionally redundant with Acbp, and expression of these molecules may vary in different tissues. This possibility could also explain the difference between yeast with a targeted deletion of the Acbp ortholog, where there is a dramatic increase in long-chain fatty acyl-CoA esters (Schjerling et al., 1996), and nm1054 mouse hair, where the vast majority of lipids are synthesized at normal levels and have correct fatty acyl chain lengths. Alternatively, it is possible that loss of Acbp produces a cell-autonomous defect in mouse skin, whereas other tissues may compensate for a loss of Acbp by taking up lipids or other molecules produced elsewhere through redundant pathways. Such molecules may not be in sufficient supply in skin, necessitating the requirement for endogenous Acbp. This hypothesis could explain the apparently paradoxical findings that Acbp is essential in cell culture (Faergeman and Knudsen, 2002) and the whole animal lacking the gene survives.
At the present time, no human disorders have been described that resemble the hair and skin phenotype seen in nm1054 mice, and no related lipid metabolism disorders have been mapped near ACBP. However, an increased understanding of the role of Acbp in cutaneous development and maintenance could play a pivotal role in future studies of human skin disease pathophysiology and therapy.
MATERIALS AND METHODS
Mice
The nm1054 mutation was maintained on both the C57BL/6J (B6) and the 129S6/SvEvTac (129) backgrounds as previously described (Ohgami et al., 2005a). All phenotypic analysis was performed on B6129F1 males at 8 or 24 weeks. All animal procedures were approved by the Animal Care and Use Committee at Children’s Hospital Boston.
Transgenic rescue
BAC transgenic animals were generated as previously described (Ohgami et al., 2005b). Transgenic mice were identified by BAC end-specific PCR, as well as internal polymorphisms between the 129X1/SvJ transgene and the C57BL/6 background.
Histological analysis
Eight- and 24-week-old mice were euthanized, and the entire pelt and the ears were fixed in 10% buffered formalin. After 24 hours, the nose and standardized sections of skin from the lower thorax were transferred to 70% ethanol. Paws and tails were fixed in Bouin’s fixative until the bones were fully decalcified. All tissues were embedded in paraffin, sectioned, and stained with hematoxylin and eosin.
Northern analysis
A 32P-labeled Acbp probe was generated by PCR amplification of the open reading frame from EST clone BQ947297 (Invitrogen, Carlsbad, CA), which contains full-length Acbp, using primers 5’- CCTCAAGACTCAGCCAACTG-3’ and 5’- GACTCGTGGAACAAGCTGAA-3’. A multi-tissue mouse northern blot (OriGene Technologies, Inc., Rockville, MD) was prehybridized in MiracleHyb solution (Stratagene, La Jolla, CA) for 30 minutes at 65oC and hybridized in the same solution with the Acbp probe. The blot was washed twice with 2X SSC / 0.1% SDS and then twice with 0.2X SSC / 0.1% SDS, all at 65oC. Expression was detected by autoradiography.
In situ hybridization
Ten micrograms of EST clone BQ947297 (Invitrogen, Carlsbad, CA) were digested with SalI and HindIII to generate antisense and sense probes, respectively. Riboprobes were labeled using a digoxigenin RNA labeling kit (Roche Molecular Biochemicals, Indianapolis, IN). Automated in situ hybridization was performed on the Ventana Discovery System (Ventana Medical Systems, Tucson, AZ). Wild type and nm1054 skin were fixed in 4% paraformaldehyde and embedded in paraffin. Deparaffinized sections were digested with proteinase K at 37oC, hybridized at 65oC for 6 hours, and washed twice in 0.1X SSC at 75oC. The digoxigenin label was detected with a biotinylated anti-digoxigenin antibody (Biogenex, San Ramon, CA), followed by a streptavidin-alkaline phosphatase conjugate, and visualized by an NBT/BCIP substrate reaction with the BlueMap Detection Kit (Ventana Medical Systems, Tucson, AZ). Slides were counterstained with nuclear fast red (Vector Laboratories, Inc., Burlingame, CA), dehydrated, and analyzed by light microscopy.
RT PCR
RNA was isolated from mouse thymus using the RNAqueous kit (Ambion, Austin, TX). 0.5μg RNA was primed with an oligo-dT primer and reverse transcribed using the SuperScript first strand synthesis kit (Invitrogen, Carlsbad, CA). BAB29121 was amplified from reverse transcribed cDNA using primers 5’-CTCATCCGTTCATTTGAGCA-3’ and 5’-CAGGACGAGATGGGATTAGC-3’. Amplification products were visualized by agarose gel electrophoresis.
Lipid isolation
Lipids were derived from plucked fur, sections of shaved skin, and sections of liver from eight- and 24-week old male mice fasted for four hours. Lipids were extracted serially with choloform:methanol 2:1 (v/v), chloroform:methanol 1:1, and chloroform:methanol 1:2, each for one hour at room temperature. After each extraction, the samples were centrifuged at 2,000 X g for five minutes. The supernatants containing the lipids were pooled, evaporated under a nitrogen stream, and solvated in cholorform.
Thin layer chromatography
Polar and non-polar lipids were separated by thin layer chromatography (TLC) using silica plates (Scientific Adsorbents, Inc., Atlanta, GA). Equal amounts of lipids extracted from plucked hair, skin, or liver of wild type, nm1054, and BAC 486B9 transgenic nm1054 mice were spotted onto TLC plates. For polar lipids, plates were developed with chloroform:methanol:water 60:35:5 (v/v/v). Non-polar lipids were separated and analyzed as previously described (Downing, 1968). Briefly, after development with hexane to the top of the plate, the plate was dried, redeveloped to the same point with benzene, dried, and developed again with hexane:diethyl ether:acetic acid 70:30:1 (v/v/v) to the midpoint of the plate. TLC bands were visualized either by spraying the plate with 3% cupric acetate (w/v) in 8% phosphoric acid (v/v), followed by charring at 180oC, or by a non-destructive method modified from Plekhanov (Plekhanov, 1999). Briefly, the plate was sprayed with 1M ammonium acetate and then submerged in a 1M ammonium acetate solution saturated with naphthol blue black (Sigma-Aldrich, St. Louis, MO) and dried. Bands were scraped and extracted three times with 5 ml chloroform:methanol 2:1 (v/v).
High performance liquid chromatography – mass spectrometry
Equal amounts of polar lipids were separated by high performance liquid chromatography – mass spectrometry using a method modified from Matsunaga et al. (Matsunaga et al., 2004). A Monochrome Diol column was coupled online to an LCQ Advantage ion-trap mass spectrometer equipped with an electrospray ionization source. Mobile phase A was hexane:isopropanol 60:40 (v/v) containing 0.1% formic acid, 0.05% ammonium hydroxide, and 0.05% triethylamine. Mobile phase B was methanol containing 0.1% formic acid, 0.05% ammonium hydroxide, and 0.05% triethylamine. Using a flow rate of 0.7 ml/minute, a binary gradient started at 5% mobile phase B (B), increased linearly to 15% B in six minutes, held for 10 minutes, increased linearly to 100% B in 12 minutes, held for six minutes, and returned to 5% B in two minutes. Eluants were analyzed by UV absorbance and direct online mass spectrometry.
Supplementary Material
Acknowledgments
We gratefully thank James Edwards and Tonora Archibald in the Pathology Department’s histology core facility at Children’s Hospital Boston, as well as Yu Yang in the Dana Farber – Partners Cancer Center in situ core facility, for their technical assistance and expertise. We also thank Nancy Andrews and members of the Andrews and Fleming labs for their ongoing suggestions. This work was supported by the Pew Foundation Scholars in the Biomedical Sciences Program (M.D.F. and D.B.M.), the Mizutani Foundation for Glycoscience (D.B.M.), and the National Institutes of Health (HL074247 for M.D.F. and AR48632 and AI49313 for D.B.M.). L.L. was supported in part by an NIH postdoctoral NRSA training grant (HL007574.23).
ABBREVIATIONS
- Acbp
Acyl-CoA binding protein
- B6
C57BL/6J
- 129
129S6/SvEvTac
- PSU
pilosebaceous unit
- TLC
thin-layer chromatography
- PPAR
peroxisome proliferator-activated receptor
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
LOCATION OF WORK: Boston, Massachusetts, USA
References
- Alho H, Vaalasti A, Podkletnova I, Rechardt L. Expression of diazepam-binding inhibitor peptide in human skin: an immunohistochemical and ultrastructural study. J Invest Dermatol. 1993;101:800–3. doi: 10.1111/1523-1747.ep12371698. [DOI] [PubMed] [Google Scholar]
- Bovolin P, Schlichting J, Miyata M, Ferrarese C, Guidotti A, Alho H. Distribution and characterization of diazepam binding inhibitor (DBI) in peripheral tissues of rat. Regul Pept. 1990;29:267–81. doi: 10.1016/0167-0115(90)90089-f. [DOI] [PubMed] [Google Scholar]
- Carninci P, Hayashizaki Y. High-efficiency full-length cDNA cloning. Methods Enzymol. 1999;303:19–44. doi: 10.1016/s0076-6879(99)03004-9. [DOI] [PubMed] [Google Scholar]
- Chen HC, Smith SJ, Tow B, Elias PM, Farese RV., Jr Leptin modulates the effects of acyl CoA:diacylglycerol acyltransferase deficiency on murine fur and sebaceous glands. J Clin Invest. 2002;109:175–81. doi: 10.1172/JCI13880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di-Poi N, Michalik L, Desvergne B, Wahli W. Functions of peroxisome proliferator-activated receptors (PPAR) in skin homeostasis. Lipids. 2004;39:1093–9. doi: 10.1007/s11745-004-1335-y. [DOI] [PubMed] [Google Scholar]
- Downing DT. Photodensitometry in the thin-layer chromatographic analysis of neutral lipids. J Chromatogr. 1968;38:91–9. doi: 10.1016/0021-9673(68)85011-3. [DOI] [PubMed] [Google Scholar]
- Faergeman NJ, Knudsen J. Role of long-chain fatty acyl-CoA esters in the regulation of metabolism and in cell signalling. Biochem J. 1997;323 :1–12. doi: 10.1042/bj3230001. Pt 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faergeman NJ, Knudsen J. Acyl-CoA binding protein is an essential protein in mammalian cell lines. Biochem J. 2002;368:679–82. doi: 10.1042/BJ20021413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraris C, Bernard BA, Dhouailly D. Adult epidermal keratinocytes are endowed with pilosebaceous forming abilities. Int J Dev Biol. 1997;41:491–8. [PubMed] [Google Scholar]
- Forman BM, Chen J, Evans RM. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc Natl Acad Sci U S A. 1997;94:4312–7. doi: 10.1073/pnas.94.9.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervois P, Torra IP, Fruchart JC, Staels B. Regulation of lipid and lipoprotein metabolism by PPAR activators. Clin Chem Lab Med. 2000;38:3–11. doi: 10.1515/CCLM.2000.002. [DOI] [PubMed] [Google Scholar]
- Hall AM, Wiczer BM, Herrmann T, Stremmel W, Bernlohr DA. Enzymatic properties of purified murine fatty acid transport protein 4 and analysis of acyl-CoA synthetase activities in tissues from FATP4 null mice. J Biol Chem. 2005;280:11948–54. doi: 10.1074/jbc.M412629200. [DOI] [PubMed] [Google Scholar]
- Helledie T, Antonius M, Sorensen RV, Hertzel AV, Bernlohr DA, Kolvraa S, et al. Lipid-binding proteins modulate ligand-dependent trans-activation by peroxisome proliferator-activated receptors and localize to the nucleus as well as the cytoplasm. J Lipid Res. 2000;41:1740–51. [PubMed] [Google Scholar]
- Helledie T, Grontved L, Jensen SS, Kiilerich P, Rietveld L, Albrektsen T, et al. The gene encoding the Acyl-CoA-binding protein is activated by peroxisome proliferator-activated receptor gamma through an intronic response element functionally conserved between humans and rodents. J Biol Chem. 2002a;277:26821–30. doi: 10.1074/jbc.M111295200. [DOI] [PubMed] [Google Scholar]
- Helledie T, Jorgensen C, Antonius M, Krogsdam AM, Kratchmarova I, Kristiansen K, et al. Role of adipocyte lipid-binding protein (ALBP) and acyl-coA binding protein (ACBP) in PPAR-mediated transactivation. Mol Cell Biochem. 2002b;239:157–64. [PubMed] [Google Scholar]
- Herrmann T, van der Hoeven F, Grone HJ, Stewart AF, Langbein L, Kaiser I, et al. Mice with targeted disruption of the fatty acid transport protein 4 (Fatp 4, Slc27a4) gene show features of lethal restrictive dermopathy. J Cell Biol. 2003;161:1105–15. doi: 10.1083/jcb.200207080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herron BJ, Bryda EC, Heverly SA, Collins DN, Flaherty L. Scraggly, a new hair loss mutation on mouse chromosome 19. Mamm Genome. 1999;10:864–9. doi: 10.1007/s003359901105. [DOI] [PubMed] [Google Scholar]
- Hertz R, Berman I, Bar-Tana J. Transcriptional activation by amphipathic carboxylic peroxisomal proliferators is induced by the free acid rather than the acyl-CoA derivative. Eur J Biochem. 1994;221:611–5. doi: 10.1111/j.1432-1033.1994.tb18773.x. [DOI] [PubMed] [Google Scholar]
- Hirsch D, Stahl A, Lodish HF. A family of fatty acid transporters conserved from mycobacterium to man. Proc Natl Acad Sci U S A. 1998;95:8625–9. doi: 10.1073/pnas.95.15.8625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Atshaves BP, Frolov A, Kier AB, Schroeder F. Acyl-coenzyme a binding protein expression alters liver fatty acyl-coenzyme a metabolism. Biochemistry. 2005;44:10282–97. doi: 10.1021/bi0477891. [DOI] [PubMed] [Google Scholar]
- Hunt MC, Nousiainen SE, Huttunen MK, Orii KE, Svensson LT, Alexson SE. Peroxisome proliferator-induced long chain acyl-CoA thioesterases comprise a highly conserved novel multi-gene family involved in lipid metabolism. J Biol Chem. 1999;274:34317–26. doi: 10.1074/jbc.274.48.34317. [DOI] [PubMed] [Google Scholar]
- Kim UJ, Birren BW, Slepak T, Mancino V, Boysen C, Kang HL, et al. Construction and characterization of a human bacterial artificial chromosome library. Genomics. 1996;34:213–8. doi: 10.1006/geno.1996.0268. [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Sundseth SS, Jones SA, Brown PJ, Wisely GB, Koble CS, et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc Natl Acad Sci U S A. 1997;94:4318–23. doi: 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen J, Jensen MV, Hansen JK, Faergeman NJ, Neergaard TB, Gaigg B. Role of acylCoA binding protein in acylCoA transport, metabolism and cell signaling. Mol Cell Biochem. 1999;192:95–103. doi: 10.1007/978-1-4615-4929-1_11. [DOI] [PubMed] [Google Scholar]
- Knudsen J, Neergaard TB, Gaigg B, Jensen MV, Hansen JK. Role of acyl-CoA binding protein in acyl-CoA metabolism and acyl-CoA-mediated cell signaling. J Nutr. 2000;130:294S–8S. doi: 10.1093/jn/130.2.294S. [DOI] [PubMed] [Google Scholar]
- Kota BP, Huang TH, Roufogalis BD. An overview on biological mechanisms of PPARs. Pharmacol Res. 2005;51:85–94. doi: 10.1016/j.phrs.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Krey G, Braissant O, L’Horset F, Kalkhoven E, Perroud M, Parker MG, et al. Fatty acids, eicosanoids, and hypolipidemic agents identified as ligands of peroxisome proliferator-activated receptors by coactivator-dependent receptor ligand assay. Mol Endocrinol. 1997;11:779–91. doi: 10.1210/mend.11.6.0007. [DOI] [PubMed] [Google Scholar]
- Mandrup S, Jepsen R, Skott H, Rosendal J, Hojrup P, Kristiansen K, et al. Effect of heterologous expression of acyl-CoA-binding protein on acyl-CoA level and composition in yeast. Biochem J. 1993;290 :369–74. doi: 10.1042/bj2900369. Pt 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga I, Bhatt A, Young DC, Cheng TY, Eyles SJ, Besra GS, et al. Mycobacterium tuberculosis pks12 produces a novel polyketide presented by CD1c to T cells. J Exp Med. 2004;200:1559–69. doi: 10.1084/jem.20041429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen J, Knudsen J. Acyl-CoA-binding protein from cow. Binding characteristics and cellular and tissue distribution. Biochem J. 1987;248:709–14. doi: 10.1042/bj2480709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki M, Man WC, Ntambi JM. Targeted disruption of stearoyl-CoA desaturase1 gene in mice causes atrophy of sebaceous and meibomian glands and depletion of wax esters in the eyelid. J Nutr. 2001;131:2260–8. doi: 10.1093/jn/131.9.2260. [DOI] [PubMed] [Google Scholar]
- Mocchetti I, Einstein R, Brosius J. Putative diazepam binding inhibitor peptide: cDNA clones from rat. Proc Natl Acad Sci U S A. 1986;83:7221–5. doi: 10.1073/pnas.83.19.7221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocchetti I, Santi MR. Diazepam binding inhibitor peptide: cloning and gene expression. Neuropharmacology. 1991;30:1365–71. doi: 10.1016/s0028-3908(11)80003-1. [DOI] [PubMed] [Google Scholar]
- Moulson CL, Martin DR, Lugus JJ, Schaffer JE, Lind AC, Miner JH. Cloning of wrinkle-free, a previously uncharacterized mouse mutation, reveals crucial roles for fatty acid transport protein 4 in skin and hair development. Proc Natl Acad Sci U S A. 2003;100:5274–9. doi: 10.1073/pnas.0431186100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgami RS, Campagna DR, Antiochos B, Wood EB, Sharp JJ, Barker JE, et al. nm1054, a spontaneous, recessive, hypochromic, microcytic anemia mutation in the mouse. Blood. 2005a;106:3625–31. doi: 10.1182/blood-2005-01-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgami RS, Campagna DR, Greer EL, Antiochos B, McDonald A, Chen J, et al. Identification of a ferrireductase required for efficient transferrin-dependent iron uptake in erythroid cells. Nat Genet. 2005b;37:1264–9. doi: 10.1038/ng1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima H, Rochat A, Kedzia C, Kobayashi K, Barrandon Y. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell. 2001;104:233–45. doi: 10.1016/s0092-8674(01)00208-2. [DOI] [PubMed] [Google Scholar]
- Owens GP, Sinha AK, Sikela JM, Hahn WE. Sequence and expression of the murine diazepam binding inhibitor. Brain Res Mol Brain Res. 1989;6:101–8. doi: 10.1016/0169-328x(89)90043-0. [DOI] [PubMed] [Google Scholar]
- Plekhanov AY. Rapid staining of lipids on thin-layer chromatograms with amido black 10B and other water-soluble stains. Anal Biochem. 1999;271:186–7. doi: 10.1006/abio.1999.4127. [DOI] [PubMed] [Google Scholar]
- Rasmussen JT, Borchers T, Knudsen J. Comparison of the binding affinities of acyl-CoA-binding protein and fatty-acid-binding protein for long-chain acyl-CoA esters. Biochem J. 1990;265:849–55. doi: 10.1042/bj2650849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen JT, Faergeman NJ, Kristiansen K, Knudsen J. Acyl-CoA-binding protein (ACBP) can mediate intermembrane acyl-CoA transport and donate acyl-CoA for beta-oxidation and glycerolipid synthesis. Biochem J. 1994;299 :165–70. doi: 10.1042/bj2990165. Pt 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen JT, Rosendal J, Knudsen J. Interaction of acyl-CoA binding protein (ACBP) on processes for which acyl-CoA is a substrate, product or inhibitor. Biochem J. 1993;292 :907–13. doi: 10.1042/bj2920907. Pt 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AJ, Jahoda CA. Cultured dermal papilla cells induce follicle formation and hair growth by transdifferentiation of an adult epidermis. Development. 1992;115:587–93. doi: 10.1242/dev.115.2.587. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, et al. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4:611–7. doi: 10.1016/s1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- Rosendal J, Ertbjerg P, Knudsen J. Characterization of ligand binding to acyl-CoA-binding protein. Biochem J. 1993;290 :321–6. doi: 10.1042/bj2900321. Pt 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfield RL, Deplewski D, Greene ME. Peroxisome proliferator-activated receptors and skin development. Horm Res. 2000;54:269–74. doi: 10.1159/000053270. [DOI] [PubMed] [Google Scholar]
- Rosenfield RL, Kentsis A, Deplewski D, Ciletti N. Rat preputial sebocyte differentiation involves peroxisome proliferator-activated receptors. J Invest Dermatol. 1999;112:226–32. doi: 10.1046/j.1523-1747.1999.00487.x. [DOI] [PubMed] [Google Scholar]
- Schadinger SE, Bucher NL, Schreiber BM, Farmer SR. PPARgamma2 regulates lipogenesis and lipid accumulation in steatotic hepatocytes. Am J Physiol Endocrinol Metab. 2005;288:E1195–205. doi: 10.1152/ajpendo.00513.2004. [DOI] [PubMed] [Google Scholar]
- Schjerling CK, Hummel R, Hansen JK, Borsting C, Mikkelsen JM, Kristiansen K, et al. Disruption of the gene encoding the acyl-CoA-binding protein (ACB1) perturbs acyl-CoA metabolism in Saccharomyces cerevisiae. J Biol Chem. 1996;271:22514–21. doi: 10.1074/jbc.271.37.22514. [DOI] [PubMed] [Google Scholar]
- Smith SA. Peroxisome proliferator-activated receptors and the regulation of mammalian lipid metabolism. Biochem Soc Trans. 2002;30:1086–90. doi: 10.1042/bst0301086. [DOI] [PubMed] [Google Scholar]
- Stahl A, Hirsch DJ, Gimeno RE, Punreddy S, Ge P, Watson N, et al. Identification of the major intestinal fatty acid transport protein. Mol Cell. 1999;4:299–308. doi: 10.1016/s1097-2765(00)80332-9. [DOI] [PubMed] [Google Scholar]
- Taylor G, Lehrer MS, Jensen PJ, Sun TT, Lavker RM. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell. 2000;102:451–61. doi: 10.1016/s0092-8674(00)00050-7. [DOI] [PubMed] [Google Scholar]
- Tvrdik P, Westerberg R, Silve S, Asadi A, Jakobsson A, Cannon B, et al. Role of a new mammalian gene family in the biosynthesis of very long chain fatty acids and sphingolipids. J Cell Biol. 2000;149:707–18. doi: 10.1083/jcb.149.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerberg R, Tvrdik P, Unden AB, Mansson JE, Norlen L, Jakobsson A, et al. Role for ELOVL3 and fatty acid chain length in development of hair and skin function. J Biol Chem. 2004;279:5621–9. doi: 10.1074/jbc.M310529200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


