Abstract
Neurons in the avian cochlear nucleus, nucleus magnocellularis (NM), are highly sensitive to manipulations of afferent input, and removal of afferent activity through cochlear ablation results in the death of approximately 20-40% of ipsilateral NM neurons. The intracellular cascades that determine whether an individual NM neuron will die or survive are not fully understood. One early event observed in NM following deafferentation is a rapid rise in intracellular calcium concentration. In most cellular systems, the activity of calcium-binding proteins is believed to accommodate calcium influx. The calcium-binding protein, neuronal calcium sensor-1 (NCS-1), is an intracellular neuronal calcium sensor belonging to the EF-hand superfamily. NCS-1 has been implicated in calcium-dependent regulation of signaling cascades. To evaluate NCS-1 action in NM neurons, the localization of NCS-1 protein was examined. Double-label immunofluorescence experiments revealed that NCS-1 expression is evident in both the presynaptic nerve terminal and postsynaptic NM neuron. The postsynaptic expression of NCS-1 typically appears to be closely associated with the cell membrane. This close proximity of NCS-1 to the postsynaptic membrane could allow NCS-1 to function as a modulator of postsynaptic signaling events. Following deafferentation, NM neurons were more likely to show diffuse cytoplasmic NCS-1 labeling. This increase in the number of cells showing diffuse cytoplasmic labeling was observed 12 and 24 h following cochlea ablation, but was not observed 4 days following surgery. This activity-dependent regulation of NCS-1 subcellular localization suggests it may be associated with, or influenced by, processes important for the survival of NM neurons.
Keywords: deafferentation, nucleus magnocellularis, calcium binding proteins, frequenin, auditory system, cell death
Manipulations of afferent input have dramatic consequences on the metabolism and survival of postsynaptic neurons in the CNS. These consequences are particularly pronounced in developing sensory systems (e.g. Hubel and Wiesel 1970; Van der Loos and Woolsey, 1973; Galli-Resta et al., 1993; Brunjes, 1994; Zhang and Poo, 2001). In the developing avian brainstem auditory system, the elimination of afferent input modifies the morphology, metabolism, gene expression, and survival of postsynaptic neurons (Rubel et al., 1990; Wilkinson et al., 2002). However, the intracellular cascade of events and the possible intracellular factors that modulate the effects of deafferentation have not been fully elucidated.
In the avian auditory system, the eighth cranial nerve provides excitatory drive to neurons in the ipsilateral cochlear nucleus, nucleus magnocellularis (NM). Removal of auditory nerve activity through cochlear ablation results in the death of 20–40% of the ipsilateral NM neurons, with the surviving population of neurons displaying marked changes in both morphology and physiology (for review see Rubel et al., 1990). One early event observed in NM neurons following deafferentation is a rapid rise in the intracellular calcium concentration [Ca2+]i (Zirpel et al., 1995). This rise in [Ca2+]i has been shown to be directly related to the loss of activity at the level of metabotropic glutamate receptors (mGluRs) (Zirpel and Rubel, 1996). Normally, mGluRs linked to the protein kinase C (PKC) and protein kinase A pathways regulate calcium homeostasis in NM neurons (Zirpel et al., 1998). Following deafferentation, the loss of mGluR activity leads to a rise in [Ca2+]i, followed by degradation of polyribosomes and eventual cell death (Hyson, 1997, 1998; Hyson and Rubel, 1989).
Brainstem auditory neurons have high rates of spontaneous activity, reaching up to 100 Hz even in the absence of acoustic stimulation (Warchol and Dallos, 1990). One might expect that these high rates of activity would allow substantial levels of Ca2+ to enter these cells through voltage-dependent calcium channels. Since auditory neurons are susceptible to large activity-dependent influxes of Ca2+, these neurons likely have compensatory mechanisms that respond to high Ca2+ influxes in order to maintain calcium homeostasis. In a variety of systems, maintenance of calcium homeostasis and Ca2+-dependent signaling effects are mediated by a large family of intracellular proteins known as calcium-binding proteins (Baimbridge et al., 1992). In the avian brainstem auditory system, several of these proteins have been studied including: calbindin D28k, parvalbumin, and calretinin (Rogers, 1987, 1989; Braun, 1990). Of these, calretinin has been the most thoroughly examined (Parks et al., 1997; Kubke et al., 1999; Hack et al., 2000). However, calretinin does not show a dependence on eighth-nerve activity for either its mRNA expression or its constitutive protein expression (Parks et al., 1997; Stack and Code, 2000). The potential activity-dependent regulation of other calcium-binding proteins has not been examined.
Neuronal calcium sensors (NCSs) are a subfamily of intracellular EF-hand calcium-binding proteins predominantly expressed in neurons (Braunewell and Gundelfinger, 1999; Burgoyne and Weiss, 2001). These proteins are highly conserved across species including Drosophila (Pongs et al., 1993), Caenorhabditis elegans (De Castro et al., 1995), human (Bourne et al., 2001), rodents (Olafsson et al., 1997; Martone et al., 1999), crustaceans (Jeromin et al., 1999), avian (Nef et al., 1995), Xenopus (Olafsson et al., 1995), and yeast (Hendricks et al., 1999). NCS proteins exhibit a common structural motif that includes a high affinity EF-hand binding domain and an N-terminal myristoylation site (Braunewell and Gundelfinger, 1999). NCS-1 (frequenin), a member of this protein family, has been implicated in the facilitation of neurotransmitter release (Pongs et al., 1993; Rivosecchi et al., 1994; Olafsson et al., 1995; Chen et al., 2001), receptor phosphorylation (Nef et al., 1995), phosphatidylinositol metabolism (Bartlett et al., 2000; Weisz et al., 2000; Zhao et al., 2001), and can act as a substitute for calmodulin (Schaad et al., 1996). Clearly, NCS-1 can act on numerous substrates and, consequently, this molecule may serve different functions in different populations of neurons. Given the importance of calcium homeostasis in the survival of NM neurons, we sought to examine the localization of NCS-1 in the avian brainstem auditory system.
Since NCS-1 has been shown to be localized in both presynaptic and postsynaptic cellular compartments (Olafsson et al., 1997; Martone et al., 1999), experiments were designed to identify the localization of NCS-1 in NM neurons. We present evidence here that the NCS-1 protein is localized in both the presynaptic eighth nerve terminals and the postsynaptic compartment of NM neurons. Given the postsynaptic localization of NCS-1, we examined whether elimination of afferent activity could alter the expression pattern of NCS-1 in NM neurons.
EXPERIMENTAL PROCEDURES
Immunocytochemistry
Tissue sections were processed for NCS-1 immunocytochemistry using an affinity-purified antisera against mouse/rat NCS-1 (44162 and 44163; Werle et al., 2000). Previous Western blot analysis of the antiserum used in these experiments has revealed that this NCS-1 antiserum specifically recognizes chick NCS-1 (Bergmann et al., 2002). Posthatch chicks (7–10 days old) were transcardially perfused with 0.9% saline followed by ice-cold 4% paraformaldehyde. Brainstems were blocked and postfixed overnight in 20% sucrose in 4% paraformaldehyde at 4 °C. For cryosectioning, brainstems were rapidly frozen in 2-methylbutane and embedded in Tissue-Tek OCT compound (Fisher Scientific, Pittsburgh, PA, USA). Sections (20 μm) were free-floated into phosphate-buffered saline (PBS) and preincubated in normal goat serum followed by an overnight incubation in the NCS-1 antiserum (1:500). Sections were then incubated in goat anti-rabbit secondary antibody (Vector Laboratories, Burlingame, CA, USA) and detected through the avidin–biotin–peroxidase complex (ABC kit, Vector Laboratories). Peroxidase activity was visualized with diaminobenzidine (Kirkegaard and Perry Laboratories, Gaithersburg, MD, USA). Free-floating sections were mounted on gelatin-subbed slides, dehydrated, and coverslipped. Controls included running sections from each subject in pre-immune serum or omitting the primary antibody; all controls were negative for immunoreactivity.
Double-labeled immunofluorescence
In order to determine NCS-1 localization, double-labeling immunocytochemistry was performed (n = 3) in which co-localization of NCS-1 with a presynaptic marker was examined using laser scanning confocal microscopy. Synaptic vesicle protein 2 (SV2; 1:500) was used as a marker for presynaptic terminal localization. Free-floating sections were collected in PBS and then simultaneously incubated in both primary antibodies (1:500) overnight at room temperature. Sections were then incubated in two secondary antibodies: goat anti-rabbit Cy3 (Jackson Immunoresearch Laboratory, West Grove, PA, USA) for NCS-1 and horse anti-mouse FITC (1:3000; Vector Laboratories) for SV2. Washed tissue sections were mounted with Vectashield (Vector Laboratories). All images of sections processed for double-labeled immunofluorescence were obtained on a laser scanning confocal microscope (Zeiss 510).
Animals and surgical procedures
Unilateral cochlea removal surgery was performed on 7–10-day-old posthatch chicks (Ross × Ross) (Born and Rubel, 1985). The procedures used in these experiments were approved by the Animal Care and Use Committee at The Florida State University and conform to the guidelines set forth by the National Institutes of Health. All efforts were made to minimize the number of animals used and potential suffering. Briefly, the subject was anesthetized with Halothane, and the ear canal widened with a small incision. The right tympanic membrane was punctured followed by the removal of the columella (middle ear ossicle) and the basilar papilla through the oval window. The middle ear cavity was then packed with Gelfoam and the external incision closed with surgical adhesive. This procedure eliminates afferent drive to neurons in the ipsilateral NM, while innervation to the contralateral NM remains intact (Born et al., 1991), thus providing a within-subject comparison between NM neurons on the deafferented and intact sides of the brainstem. Subjects were killed at 6 h (n = 5), 12 h (n = 3), 18 h (n = 6), 24 h (n = 6), or 4 days (n = 3) following cochlea removal and transcardially perfused with 0.9% saline followed by ice-cold 4% paraformaldehyde. Tissue was processed for NCS-1 immunocytochemistry as described above. Labeling of NM neurons was compared on the deafferented and intact sides of the same tissue sections. Several sections (average = 8) were analyzed from each subject. The number of NM neurons showing diffuse cytoplasmic labeling (NCS-1 positive) was counted on both sides of each section. To assure confidence in the cell-counting procedure, cell counts for NCS-1-positive neurons were done by two independent observers. The counts obtained by the two observers were highly correlated (r = 0.99). The difference in the number of NM neurons showing a diffuse cytoplasmic expression pattern of NCS-1 on the deafferented and intact sides of the tissue sections were compared using analysis of variance and paired t-tests using a criteria of P < 0.05 for rejection of the null hypothesis.
RESULTS
Localization of NCS-1 in NM neurons
Light microscopic analysis of peroxidase-stained tissue sections as well as tissue processed for immunofluorescence revealed a “ringing” appearance around most NM cell bodies (Fig. 1A; Fig. 2). Control sections (not shown) processed with preabsorbed antibody, no primary antibody, or preimmune serum resulted in minimal background staining and an absence of the “ringed” appearance. Since NM neurons are virtually adendritic and are contacted by large calyx-type terminals, the ringed appearance could be attributable to either presynaptic or postsynaptic labeling. To further examine the location of NCS-1 protein, the expression pattern of NCS-1 was compared with the expression pattern of a presynaptic protein, SV2, by superimposing pairs of laser confocal photomicrographs. Double-label immunofluorescence revealed some colocalization of NCS-1 with the expression pattern of SV2, indicating the presence of NCS-1 in the presynaptic (eighth nerve) terminal, but NCS-1 expression was also clearly evident within the postsynaptic NM neuron (Fig. 1C).
Fig. 1.
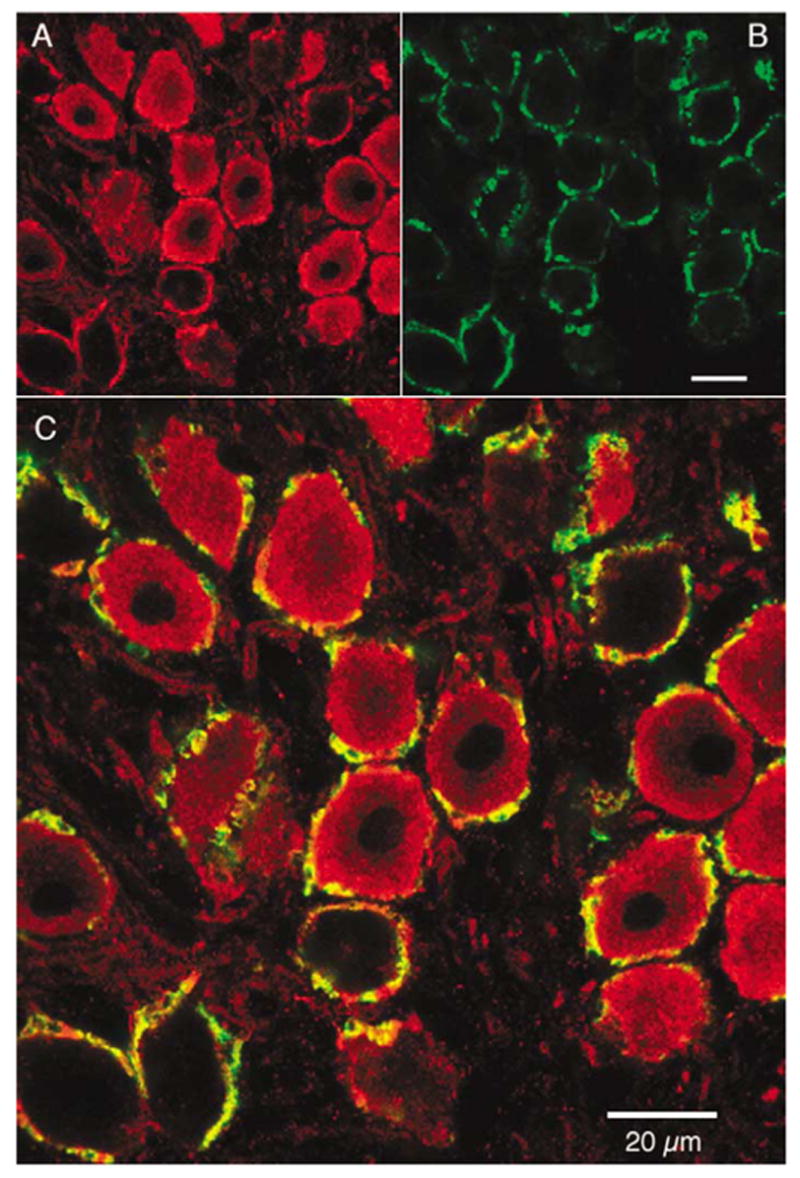
Pre-and Post-synaptic localization of neuronal calcium sensor (NCS)-1. Laser confocal photomicrographs from sections processed for double-label immunofluorescence showing: (A) immunofluorescent labeling for NCS-1 (Cy3/red), (B) immunofluorescent labeling of the presynaptic terminal marker synaptic vesicle protein 2 (SV2) (FITC/green) immunofluorescent labeling, and (C) both channels superimposed. The yellow coloring in the superimposed image is indicative of some co-localization of NCS-1 with the presynaptic marker SV2, which is present in auditory nerve fibers synapsing on NM neurons. As can be seen in both the single channel and the combined image, NCS-1 is also clearly present in postsynaptic nucleus magnocellularis (NM).
Fig. 2.
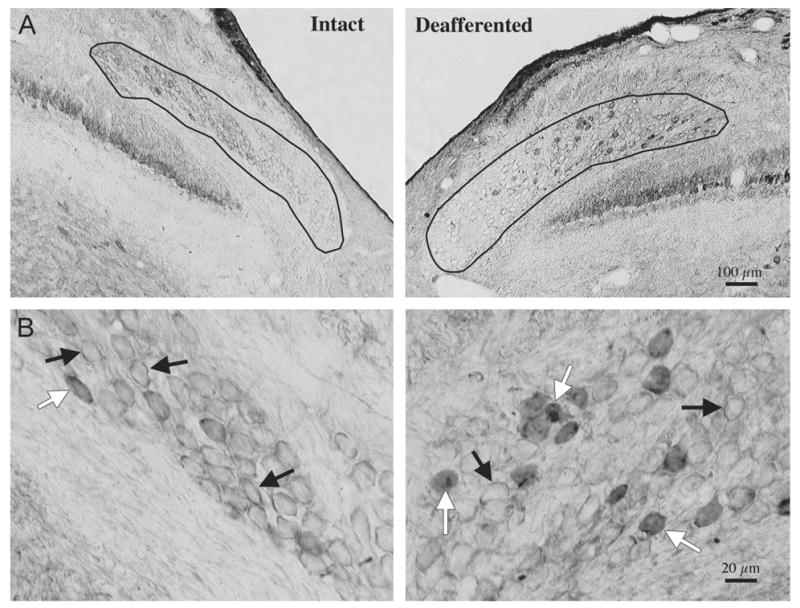
Deafferentation induced change in neuronal calcium sensor (NCS)-1 labeling. (A) Low-power photomicrographs of anti-NCS-1 peroxidase-stained tissue sections showing a “ringing” of nucleus magnocellularis (NM) cell bodies on the intact side of the brain. By 24 h following cochlea removal, an increase in the number cells with diffuse cytoplasmic NCS-1 immunolabeling can be observed in deafferented NM neurons when compared with intact NM neurons on the contralateral side of the brainstem. (B) High-power photomicrographs where white arrows point to NCS-1 diffuse labeling in the cytoplasm of deafferented NM neurons, and black arrows point to NCS-1 labeling in close proximity to the membrane.
NCS-1 immunoreactivity following deafferentation
An increase in the number of neurons with diffuse cytoplasmic NCS-1 labeling was observed in peroxidase-stained tissue sections following unilateral cochlea removal.
Fig. 2 is a representative example of immunostaining in NM 24 h after cochlea removal. The upper panels (Fig. 2A) are photomicrographs taken at a low magnification as to show the entire NM in the coronal plane. The deafferented side shows more cells with diffuse cytoplasmic labeling than the intact side. The bottom panels (Fig. 2B) are magnified views of the same tissue section. The ringed labeling pattern is observed around most neurons (black arrows) but obvious diffuse cytoplasmic labeling is observed in a subpopulation of cells (white arrows). Cell counts revealed a reliable increase in the number of neurons with diffuse cytoplasmic NCS-1 labeling on the deafferented side of the brainstem when compared with the fully innervated side of the brainstem within the same tissue section. Fig. 3 displays the mean number of cells per section that showed the diffuse cytoplasmic labeling pattern on each side of the section for brains processed at various survival times following cochlea removal.
Fig. 3.
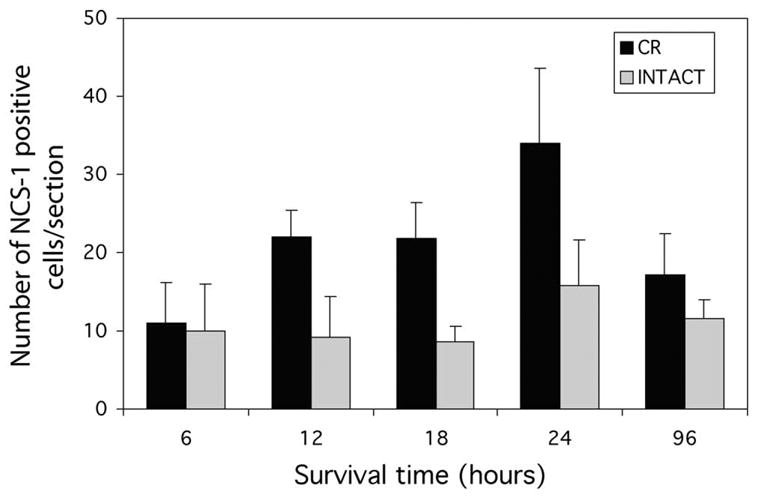
Time course of deafferentation-induced change in neuronal calcium sensor (NCS)-1 labeling. A difference between the two sides in the number of nucleus magnocellularis (NM) neurons showing diffuse cytoplasmic labeling is evident by 12 h following cochlea removal; this difference is not evident by 6 h following surgery. The increased labeling in the deafferented NM persists for at least 24 h following cochlea removal (CR), but by 96 h following surgery the difference between sides is no longer evident. Bars = S.E.M. Statistically reliable differences in the number of labeled neurons are only observed at 12, 18 and 24 h following cochlea removal.
An increased number of cells exhibiting diffuse cytoplasmic labeling following deafferentation is evident by 12 h following cochlea removal, but this increase is not observed only 6 h following the surgery. The difference between sides persists for at least 24 h following cochlea removal, but appears to wane by 4 days following surgery. Statistical analyses confirmed these visual impressions. An overall survival time×side analysis of variance (F1,16=18.228, P < 0.001) revealed a reliable difference between the two sides of the brain and individual analyses (two-tailed paired t-tests, P < 0.05) at each survival time revealed that the reliable differences between the two sides were only evident at 12, 18 and 24 h after surgery.
DISCUSSION
Localization of NCS-1 in NM neurons
The localization of NCS-1 protein within neurons of the chick brainstem is consistent with previous reports demonstrating expression of NCS-1 within the brainstem of chickens (Nef et al., 1995). We further analyzed the localization of NCS-1 specific to the cochlear nucleus (NM) of the post-hatch chick. NCS-1 was localized immunocyto-chemically to both the calyx ending (eighth nerve terminal) and the postsynaptic NM neuron. The terminal localization was verified by the colocalization of NCS-1 with SV2. These findings were consistent with other reports indicating pre- and postsynaptic localization of NCS-1 (Olafsson et al., 1997; Martone et al., 1999). Furthermore, the localization of NCS-1 is also consistent with the report by Tsujimoto et al. (2002) showing pre- and postsynaptic localization at a calyx synapse in the rat.
Changes NCS-1 postsynaptic localization following deafferentation
In immunoperoxidase-stained tissue sections, NCS-1 labeling of normally innervated NM shows a “ringed” pattern around most NM neurons. This suggests that NCS-1 is closely associated with the cell membrane or with an organelle that is concentrated in close proximity to the cell membrane of healthy neurons. Confocal microscopy shows that some of this ringed pattern may partially be attributable to localization of the antigen within the presynaptic terminal, but that there is also a concentration gradient of the antigen within the postsynaptic neuron such that greater labeling is observed nearer the cell membrane. Following deafferentation, there is an increase in the number of NM neurons showing diffuse cytoplasmic labeling for NCS-1. The cause of this increase in the number of neurons showing diffuse cytoplasmic labeling is unknown, but two possibilities are worthy of discussion. First, the cytoplasmic labeling pattern observed in a subpopulation of NM neurons could represent an increase in the transcription and translation of NCS-1 in response to deafferentation. The possibility of newly synthesized NCS-1 protein is believed to be unlikely since overall mRNA and protein synthesis is reduced across the entire extent of NM following cochlea removal (Born and Rubel, 1985; Garden et al., 1995). However, recent findings have shown that expression of some specific molecules is upregulated following cochlea removal despite the reduction in overall macromolecular synthesis (Wilkinson et al., 2002). Further studies examining the expression of NCS-1 mRNA following deafferentation would be needed to assess the likelihood of upregulated NCS-1 protein synthesis.
A second intracellular event that could potentially increase cytoplasmic NCS-1 labeling is a redistribution of existing NCS-1 within the neuron. The typical ringing pattern around most NM neurons suggests that NCS-1 is concentrated around or near the cell membrane, perhaps anchored to the membrane or a membrane-associated organelle. Deafferentation may cause the uncoupling of this anchor, or the movement of membrane-associated organelles, to produce the diffuse pattern of cytoplasmic NCS-1 labeling. This movement away from the membrane could possibly be initiated by a rise in intracellular calcium concentrations ([Ca2+]i). Ratiometric imaging studies have shown that [Ca2+]i is elevated in NM neurons on the deafferented side of the brain following cochlea removal (Zirpel et al., 1995, 1996). Some NCS family proteins, such as guanyl cyclase-activating protein 2 show binding to membranes at low Ca2+ levels, but detach from the membrane at high Ca2+ concentrations (Olshevskaya et al., 1997). If chick NCS-1 had a similar property, then one might postulate that deafferentation may cause NCS-1 to become uncoupled from the membrane as the level of [Ca2+]i rises. However, the established time courses for changes in [Ca2+]i and the changes in NCS-1 immunolabeling do not correspond to each other. The rise in [Ca2+]i is observed as early as 1 h after deafferentation, whereas the redistribution of NCS-1 labeling is not observed until sometime between 6 and 12 h following cochlea removal. If a rise in [Ca2+]i leads to a conformational change in this calcium sensor causing it to lose its association with the plasma membrane, one would not expect this conformational change to take several hours to occur. Alternatively, perhaps NCS-1 is not directly affected by the deafferentation, but an organelle to which it is anchored is affected. Bacterial-expressed NCS-1 shows Ca2+-independent binding to membranes (McFerran et al., 1999). Consequently, it is possible that NCS-1 maintains its anchor to the membrane of an organelle that is typically associated with the plasma membrane, but this organelle becomes diffusely distributed within the cell following deafferentation. This change in distribution of labeling may be caused by slower-occurring changes in NM neurons following deafferentation, such as the disruption in protein synthesis or breakdown of the cytoskeleton (see Rubel et al., 1990).
Potential NCS-1 signaling mechanisms in NM neurons
The fact that only some NM neurons on the deafferented side of the brain show diffuse cytoplasmic labeling is intriguing because only a subpopulation of neurons dies in this nucleus following cochlea ablation. Additionally, the time course of the increase in the number of cells showing diffuse cytoplasmic labeling is correlated with the time course for neuronal death following deafferentation. Other events that are proposed to segregate NM neurons into dying and surviving populations, such as assays for ribosomal integrity (Steward and Rubel, 1985; Rubel et al., 1991; Garden et al., 1994), are observed within the first day after cochlea ablation and the NM neurons degenerate within the 4-day period that was examined in this study (Born and Rubel, 1985). Since the changes in NCS-1 immunoreactivity are also seen in the first 24 h following deafferentation and are absent after the dying NM neurons have degenerated, the subcellular localization of NCS-1 may be associated with or influenced by those processes important for the survival of NM neurons. It is not clear whether those neurons showing diffuse cytoplasmic labeling are destined to die, but it is clear that diffuse cytoplasmic labeling for NCS-1 is not a definitive sign of cell death because some cells on the intact side of the brain also show diffuse cytoplasmic labeling. There is no evidence that cells on the intact side of the brain die in the post-hatch chicken and cell counts have shown this population to be stable over the age range used in this study (Born and Rubel, 1985).
The biochemical function NCS-1 might play in normal NM neurons is still unclear; however, based on some of the identified substrates of NCS-1 and what is known about the physiology of NM neurons, we can provide two intriguing speculations. First, since NCS-1 distribution within NM neurons suggests a close association with the membrane, it is likely that NCS-1 could function as a modulator of postsynaptic signaling events in NM neurons. NCS-1 may possibly act to modulate second-messenger signaling in NM through multiple pathways. The mGluRs found in NM neurons have been shown to be linked to at least two separate second-messenger signaling cascades. One pathway is linked to the hydrolysis of phosphatidylinositol and the activation of phospholipase C (PLC). This pathway results in the activation of PKC and generates IP3, which can act to release Ca2+ from internal stores. Activation of these mGluRs appears to be crucial for the survival of NM neurons (Zirpel et al., 1996; Hyson, 1998). Although NCS-1 has not been directly linked to PLC activation, an NCS-1 orthologue in yeasts (Saccharomyces cerevisiae) was shown to be essential for the activation of phosphati-dylinositol-4-kinase (Hendricks et al., 1999). This enzyme is necessary for the synthesis of polyphosphatidylinositols and consequently the maintenance of phosphainositol bisphosphate. This upstream action of NCS-1 could facilitate and maintain the essential PLC pathway in NM neurons.
A second possible role of NCS-1 is through a more direct action on the function of mGluRs and calcium homeostasis. NM neurons receive large secure synapses from the auditory nerve and are driven at high rates with spontaneous activity of 50–100 spikes per second (Warchol and Dallos, 1990). Such high rates of activity could be expected to produce potentially toxic levels of Ca2+ influx through voltage-dependent Ca2+ channels. The proximity of NCS-1 to the membrane may allow for rapid responses to local changes in Ca2+ concentrations and work to prevent Ca2+ from reaching toxic levels. It has been suggested that the role of NCS-1 is not to buffer Ca2+, but rather, Ca2+ causes a conformational change in NCS-1 that leads to an interaction with various substrates (Schaad et al., 1996). One substrate that may be relevant to the normal function of NM neurons is the G protein receptor kinases (GRKs), which have a direct influence on the function of mGluRs.
Activation of mGluRs appears to be critical for the survival of NM neurons. It is through the activation of mGluRs that high rates of driven activity do not result in cytotoxic increases in intracellular Ca2+ concentrations. However, in the continual presence of ligand, mGluRs will eventually desensitize. It appears that phosphorylation of mGluRs by GRKs causes this desensitization (Dale et al., 2000; Sallese et al., 2000a). One might expect that in the presence of constant, high levels of afferent drive, such as observed at the auditory nerve-NM synapse, the continuous exposure to glutamate will cause mGluR desensitization. Since the activation of mGluRs is crucial for maintaining calcium homeostasis, such receptor desensitization would be deadly. NCS-1, however, inhibits the activation of GRKs in a Ca2+-dependent manner (Iacovelli et al., 1999; Sallese et al., 2000b) and in this way prevents phosphorylation and desensitization of the mGluR. Consequently, NCS-1, which is localized near the postsynaptic membrane of normal NM neurons, may play a role in maintaining mGluR sensitivity in the face of constant high levels of ligand. In this way, NCS-1 may play an integral role in the survival of NM neurons (De Castro et al., 1995).
Abbreviations
- [Ca2+]i
intracellular calcium concentration
- GRK
G-protein receptor kinase
- mGluR
metabotropic glutamate receptor
- NCS
neuronal calcium sensor
- NM
nucleus magnocellularis
- PBS
phosphate-buffered saline
- PKC
protein kinase C
- PLC
phospholipase C
- SV2
synaptic vesicle protein 2
References
- Baimbridge KG, Celio MR, Rogers JH. Calcium-binding proteins in the nervous system. Trends Neurosci. 1992;15:303–308. doi: 10.1016/0166-2236(92)90081-i. [DOI] [PubMed] [Google Scholar]
- Bartlett SE, Reynolds AJ, Weible M, Jeromin A, Roder J, Hendry IA. PtdIns 4-kinasebeta and neuronal calcium sensor-1 co-localize but may not directly associate in mammalian neurons. J Neurosci Res. 2000;62:216–224. doi: 10.1002/1097-4547(20001015)62:2<216::AID-JNR6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Bergmann M, Grabs D, Roder J, Rager G, Jeromin A. Differential expression of neuronal calcium sensor-1 in the developing chick retina. J Comp Neurol. 2002;449:231–240. doi: 10.1002/cne.10302. [DOI] [PubMed] [Google Scholar]
- Born DE, Rubel EW. Afferent influences on brain stem auditory nuclei of the chicken: neuron number and size following cochlea removal. J Comp Neurol. 1985;231:435–445. doi: 10.1002/cne.902310403. [DOI] [PubMed] [Google Scholar]
- Born DE, Durham D, Rubel EW. Afferent influences on brainstem auditory nuclei of the chick: nucleus magnocellularis neuronal activity following cochlea removal. Brain Res. 1991;557:37–47. doi: 10.1016/0006-8993(91)90113-a. [DOI] [PubMed] [Google Scholar]
- Bourne Y, Dannenberg J, Pollmann V, Marchot P, Pongs O. Immunocytochemical localization and crystal structure of human frequenin (neuronal calcium sensor 1) J Biol Chem. 2001;276:11949–11955. doi: 10.1074/jbc.M009373200. [DOI] [PubMed] [Google Scholar]
- Braun K. Calcium-binding proteins in avian and mammalian central nervous system: localization, development and possible functions. Prog Histochem Cytochem. 1990;21:1–64. doi: 10.1016/s0079-6336(11)80044-6. [DOI] [PubMed] [Google Scholar]
- Braunewell KH, Gundelfinger ED. Intracellular neuronal calcium sensor proteins: a family of EF-hand calcium-binding proteins in search of a function. Cell Tissue Res. 1999;295:1–12. doi: 10.1007/s004410051207. [DOI] [PubMed] [Google Scholar]
- Brunjes PC. Unilateral naris closure and olfactory system development. Brain Res Brain Res Rev. 1994;19:146–160. doi: 10.1016/0165-0173(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Burgoyne RD, Weiss JL. The neuronal calcium sensor family of Ca2+-binding proteins. Biochem J. 2001;353:1–12. [PMC free article] [PubMed] [Google Scholar]
- Chen XL, Zhong ZG, Yokoyama S, Bark C, Meister B, Berggren PO, Roder J, Higashida H, Jeromin A. Overexpression of rat neuronal calcium sensor-1 in rodent NG108-15 cells enhances synapse formation and transmission. J Physiol. 2001;532:649–659. doi: 10.1111/j.1469-7793.2001.0649e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale LB, Bhattacharya M, Anborgh PH, Murdoch B, Bhatia M, Nakanishi S, Ferguson SS. G protein-coupled receptor kinasemediated desensitization of metabotropic glutamate receptor 1A protects against cell death. J Biol Chem. 2000;275:38213. doi: 10.1074/jbc.M006075200. [DOI] [PubMed] [Google Scholar]
- De Castro E, Nef S, Fiumelli H, Lenz SE, Kawamura S, Nef P. Regulation of rhodopsin phosphorylation by a family of neuronal calcium sensors. Biochem Biophys Res Commun. 1995;216:133–140. doi: 10.1006/bbrc.1995.2601. [DOI] [PubMed] [Google Scholar]
- Galli-Resta L, Ensini M, Fusco E, Gravina A, Margheritti B. Afferent spontaneous electrical activity promotes the survival of target cells in the developing retinotectal system of the rat. J Neurosci. 1993;13:243–250. doi: 10.1523/JNEUROSCI.13-01-00243.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garden GA, Canady KS, Lurie DI, Bothwell M, Rubel EW. A biphasic change in ribosomal conformation during transneuronal degeneration is altered by inhibition of mitochondrial, but not cytoplasmic protein synthesis. J Neurosci. 1994;14:1994–2008. doi: 10.1523/JNEUROSCI.14-04-01994.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garden GA, Redeker-DeWulf V, Rubel EW. Afferent influences on brainstem auditory nuclei of the chicken: regulation of transcriptional activity following cochlea removal. J Comp Neurol. 1995;359:412–423. doi: 10.1002/cne.903590305. [DOI] [PubMed] [Google Scholar]
- Hack NJ, Wride MC, Charters KM, Kater SB, Parks TN. Developmental changes in the subcellular localization of calretinin. J Neurosci. 2000;20:RC67. doi: 10.1523/JNEUROSCI.20-07-j0001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks KB, Wang BQ, Schnieders EA, Thorner J. Yeast homologue of neuronal frequenin is a regulator of phosphatidylino-sitol-4-OH kinase. Nat Cell Biol. 1999;1:234–241. doi: 10.1038/12058. [DOI] [PubMed] [Google Scholar]
- Hyson RL. Transneuronal regulation of ribosomes after blockade of ionotropic excitatory amino acid receptors. Brain Res. 1997;749:61–70. doi: 10.1016/s0006-8993(96)01160-2. [DOI] [PubMed] [Google Scholar]
- Hyson RL. Activation of metabotropic glutamate receptors is necessary for transneuronal regulation of ribosomes in chick auditory neurons. Brain Res. 1998;809:214–220. doi: 10.1016/s0006-8993(98)00873-7. [DOI] [PubMed] [Google Scholar]
- Hyson RL, Rubel EW. Transneuronal regulation of protein synthesis in the brain stem auditory system of the chick requires synaptic activation. J Neurosci. 1989;9:2835–2845. doi: 10.1523/JNEUROSCI.09-08-02835.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol. 1970;206:419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacovelli L, Sallese M, Mariggio S, de Blasi A. Regulation of G-protein-coupled receptor kinase subtypes by calcium sensor proteins. FASEB J. 1999;13:1–8. doi: 10.1096/fasebj.13.1.1. [DOI] [PubMed] [Google Scholar]
- Jeromin A, Shayan AJ, Msghina M, Roder J, Atwood HL. Crustacean frequenins: molecular cloning and differential localization at neuromuscular junctions. J Neurobiol. 1999;41:165–175. [PubMed] [Google Scholar]
- Kubke MF, Gauger B, Basu L, Wagner H, Carr CE. Development of calretinin immunoreactivity in the brainstem auditory nuclei of the barn owl (Tyto alba) J Comp Neurol. 1999;415:189–203. doi: 10.1002/(sici)1096-9861(19991213)415:2<189::aid-cne4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Martone ME, Edelmann VM, Ellisman MH, Nef P. Cellular and subcellular distribution of the calcium-binding protein NCS-1 in the central nervous system of the rat. Cell Tissue Res. 1999;295:395–407. doi: 10.1007/s004410051246. [DOI] [PubMed] [Google Scholar]
- McFerran BW, Weiss JL, Burgoyne RD. Neuronal Ca(2+) sensor 1: Characterization of the myristoylated protein, its cellular effects in permeabilized adrenal chromaffin cells, Ca(2+)-independent membrane association, and interaction with binding proteins, suggesting a role in rapid Ca(2+) signal transduction. J Biol Chem. 1999;274:30258–30265. doi: 10.1074/jbc.274.42.30258. [DOI] [PubMed] [Google Scholar]
- Nef S, Fiumelli H, de Castro E, Raes MB, Nef P. Identification of neuronal calcium sensor (NCS-1) possibly involved in the regulation of receptor phosphorylation. J Recept Signal Transduct Res. 1995;15:365–378. doi: 10.3109/10799899509045227. [DOI] [PubMed] [Google Scholar]
- Olafsson P, Soares HD, Herzog KH, Wang T, Morgan JI, Lu B. The Ca2+ binding protein, frequenin is a nervous system-specific protein in mouse preferentially localized in neurites. Brain Res Mol Brain Res. 1997;44:73–82. doi: 10.1016/s0169-328x(96)00188-x. [DOI] [PubMed] [Google Scholar]
- Olafsson P, Wang T, Lu B. Molecular cloning and functional characterization of the Xenopus Ca(2+)-binding protein frequenin. Proc Natl Acad Sci USA. 1995;92:8001–8005. doi: 10.1073/pnas.92.17.8001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olshevskaya EV, Hughes RE, Hurley JB, Dizhoor AM. Calcium binding, but not a calcium-myristoyl switch, controls the ability of guanylyl cyclase-activating protein GCAP-2 to regulate photoreceptor guanylyl cyclase. J Biol Chem. 1997;272:14327–14333. doi: 10.1074/jbc.272.22.14327. [DOI] [PubMed] [Google Scholar]
- Parks TN, Code RA, Taylor DA, Solum DA, Strauss KI, Jacobowitz DM, Winsky L. Calretinin expression in the chick brainstem auditory nuclei develops and is maintained independently of cochlear nerve input. J Comp Neurol. 1997;383:112–121. [PubMed] [Google Scholar]
- Pongs O, Lindemeier J, Zhu XR, Theil T, Engelkamp D, Krah-Jentgens I, Lambrecht HG, Koch KW, Schwemer J, Rivosecchi R, et al. Frequenin: a novel calcium-binding protein that modulates synaptic efficacy in the Drosophila nervous system. Neuron. 1993;11:15–28. doi: 10.1016/0896-6273(93)90267-u. [DOI] [PubMed] [Google Scholar]
- Rivosecchi R, Pongs O, Theil T, Mallart A. Implication of frequenin in the facilitation of transmitter release in Drosophila. J Physiol. 1994;474:223–232. doi: 10.1113/jphysiol.1994.sp020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JH. Calretinin: a gene for a novel calcium-binding protein expressed principally in neurons. J Cell Biol. 1987;105:1343–1353. doi: 10.1083/jcb.105.3.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JH. Two calcium-binding proteins mark many chick sensory neurons. Neuroscience. 1989;31:697–709. doi: 10.1016/0306-4522(89)90434-x. [DOI] [PubMed] [Google Scholar]
- Rubel EW, Falk PM, Canady KS, Steward O. A cellular mechanism underlying activity-dependent transneuronal degeneration: rapid but reversible destruction of neuronal ribosomes. Brain Dysfunct. 1991;4:55–74. [Google Scholar]
- Rubel EW, Hyson RL, Durham D. Afferent regulation of neurons in the brain stem auditory system. J Neurobiol. 1990;21:169–196. doi: 10.1002/neu.480210112. [DOI] [PubMed] [Google Scholar]
- Sallese M, Iacovelli L, Cumashi A, Capobianco L, Cuomo L, De Blasi A. Regulation of G protein-coupled receptor kinase subtypes by calcium sensor proteins. Biochim Biophys Acta. 2000b;1498:112–121. doi: 10.1016/s0167-4889(00)00088-4. [DOI] [PubMed] [Google Scholar]
- Sallese M, Salvatore L, D’Urbano E, Sala G, Storto M, Launey T, Nicoletti F, Knopfel T, De Blasi A. The G-protein-coupled receptor kinase GRK4 mediates homologous desensitization of metabotropic glutamate receptor 1. FASEB J. 2000a;14:2569–2580. doi: 10.1096/fj.00-0072com. [DOI] [PubMed] [Google Scholar]
- Schaad NC, De Castro E, Nef S, Hegi S, Hinrichsen R, Martone ME, Ellisman MH, Sikkink R, Rusnak F, Sygush J, Nef P. Direct modulation of calmodulin targets by the neuronal calcium sensor NCS-1. Proc Natl Acad Sci USA. 1996;93:9253–9258. doi: 10.1073/pnas.93.17.9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack KE, Code RA. Calretinin expression in the chick cochlear nucleus after deafferentation. Brain Res. 2000;873:135–139. doi: 10.1016/s0006-8993(00)02476-8. [DOI] [PubMed] [Google Scholar]
- Steward O, Rubel EW. Afferent influences on brain stem auditory nuclei of the chicken: cessation of amino acid incorporation as an antecedent to age-dependent transneuronal degeneration. J Comp Neurol. 1985;231:385–395. doi: 10.1002/cne.902310308. [DOI] [PubMed] [Google Scholar]
- Tsujimoto T, Jeromin A, Saitoh N, Roder JC, Takahashi T. Neuronal calcium sensor 1 and activity-dependent facilitation of P/Q-type calcium currents at presynaptic nerve terminals. Science. 2002;295:2276–2279. doi: 10.1126/science.1068278. [DOI] [PubMed] [Google Scholar]
- Van der Loos H, Woolsey TA. Somatosensory cortex: structural alterations following early injury to sense organs. Science. 1973;179:395–398. doi: 10.1126/science.179.4071.395. [DOI] [PubMed] [Google Scholar]
- Warchol ME, Dallos P. Neural coding in the chick cochlear nucleus. J Comp Physiol. 1990;166:721–734. doi: 10.1007/BF00240021. [DOI] [PubMed] [Google Scholar]
- Weisz OA, Gibson GA, Leung SM, Roder J, Jeromin A. Over-expression of frequenin, a modulator of phosphatidylinositol 4-kinase, inhibits biosynthetic delivery of an apical protein in polarized madindarby canine kidney cells. J Biol Chem. 2000;275:24341–24347. doi: 10.1074/jbc.M000671200. [DOI] [PubMed] [Google Scholar]
- Werle MJ, Roder J, Jeromin A. Expression of frequenin at the frog (Rana) neuromuscular junction, muscle spindle and nerve. Neurosci Lett. 2000;284:33–36. doi: 10.1016/s0304-3940(00)01004-1. [DOI] [PubMed] [Google Scholar]
- Wilkinson BL, Sadler KA, Hyson RL. Rapid deafferentation-induced upregulation of bcl-2 mRNA in the chick cochlear nucleus. Brain Res Mol Brain Res. 2002;99:67–74. doi: 10.1016/s0169-328x(02)00113-4. [DOI] [PubMed] [Google Scholar]
- Zhang LI, Poo MM. Electrical activity and development of neural circuits. Nat Neurosci. 2001;4 (suppl):1207–1214. doi: 10.1038/nn753. [DOI] [PubMed] [Google Scholar]
- Zhao X, Varnai P, Tuymetova G, Balla A, Toth ZE, Oker-Blom C, Roder J, Jeromin A, Balla T. Interaction of neuronal calcium sensor-1 (NCS-1) with phosphatidylinositol 4-kinase beta stimulates lipid kinase activity and affects membrane trafficking in COS-7 cells. J Biol Chem. 2001;276:40183–40189. doi: 10.1074/jbc.M104048200. [DOI] [PubMed] [Google Scholar]
- Zirpel L, Lachica EA, Rubel EW. Activation of a metabotropic glutamate receptor increases intracellular calcium concentrations in neurons of the avian cochlear nucleus. J Neurosci. 1995;15:214–222. doi: 10.1523/JNEUROSCI.15-01-00214.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirpel L, Rubel EW. Eighth nerve activity regulates intracellular calcium concentration of avian cochlear nucleus neurons via a metabotropic glutamate receptor. J Neurophysiol. 1996;76:4127–4139. doi: 10.1152/jn.1996.76.6.4127. [DOI] [PubMed] [Google Scholar]
- Zirpel L, Lippe WR, Rubel EW. Activity-dependent regulation of [Ca2+]i in avian cochlear nucleus neurons: roles of protein kinases A and C and relation to cell death. J Neurophysiol. 1998;79:2288–2302. doi: 10.1152/jn.1998.79.5.2288. [DOI] [PubMed] [Google Scholar]


