Summary
Activation of the protease caspase-3 is commonly thought to cause apoptotic cell death. Here we show that caspase-3 activity is regulated at postsynaptic sites in brain following stimuli associated with memory (neural activation and subsequent response habituation) instead of cell death. In the zebra finch auditory forebrain, the concentration of caspase-3 active sites increases briefly within minutes after exposure to tape-recorded birdsong. With confocal and immunoelectron microscopy, we localize the activated enzyme to dendritic spines. The activated caspase-3 protein is present even in unstimulated brain but bound to an endogenous inhibitor, BIRC4 (xIAP), suggesting a mechanism for rapid release and sequestering at specific synaptic sites. Caspase-3 activity is necessary to consolidate a persistent physiological trace of the song stimulus, as demonstrated using pharmacological interference and the zenk gene habituation assay. Thus the brain appears to have adapted a core component of cell death machinery to serve a unique role in learning and memory.
Keywords: zenk, caspase-3, apoptosis, synapoptosis, BIRC4, xIAP, zebra finch, learning, memory, birdsong, songbird, synapse modification, synaptic plasticity, habituation, electron microscopy, confocal microscopy, coimmunoprecipitation
Activation of caspase-3 is considered the terminal step in the biochemical cascade leading to apoptotic cell death (Afford and Randhawa, 2000). Caspase-3 is a protease, and it cleaves a long list of proteins that are important for the continued survival of the cell. Caspase-3 is itself activated by an irreversible internal cleavage event; in many cases, caspase-3 activation irreversibly commits a cell to death (Chang and Yang, 2000). In the mature nervous system, caspase activation has been associated with brain trauma (Davoli et al., 2002; Ferrer and Planas, 2003) and neurodegenerative disease (Mattson et al., 2001; Gamblin et al., 2003). Studies in cell culture and in vivo have shown that cell death following exposure to noxious or stressful stimuli can be suppressed by inhibition of caspase-3 (Robertson et al., 2000; Slagsvold et al., 2003), making caspase-3 a reasonable target for drugs to control neurodegenerative diseases.
Surprisingly, the caspase-3 protein is relatively abundant in neurons of the healthy adult brain (Chen et al., 1998; Namura et al., 1998) despite the fact that neurons are not turning over in most parts of the brain. This has prompted speculation that caspase might have some other novel physiological role in the nervous system, such as participation in synaptic modification (Mattson and Duan, 1999; Mattson et al., 1998). In support of this hypothesis, injection of activated caspase into single hippocampal neurons leads to cleavage of AMPA receptors and a reduced synaptic response to glutamatergic stimulation (Lu et al., 2002). Incubation of hippocampal slices with a caspase-3 inhibitor (DEVD) blocks the emergence of long-term potentiation with no apparent effect on short-term synaptic function communication (Gulyaeva et al., 2003; Kudryashov et al., 2004). Infusions of the inhibitor into rat brain have been shown to impair both spatial memory (Dash et al., 2000) and active avoidance learning (Stepanichev et al., 2005).
This evidence notwithstanding, three major issues undermine the hypothesis that caspase-3 has some role in normal synaptic functioning. First is the issue of activation. One would expect to observe a localized change in caspase-3 activity during (or soon after) a training experience. Yet prior studies have failed to detect any change in the activated form of caspase-3 associated with behavioral training, despite the effects of inhibitor infusion on animal performance (Dash et al., 2000). Second is the issue of localization. If caspase-3 acts specifically on subsets of synapses to modify them, then one would expect to observe a synaptic localization for the endogenous activity. Indeed, Mattson et al (1999) observed dendritic localization of activated caspase in cultured hippocampal neurons following bath application of glutamate. However, synaptic localization has not been demonstrated in vivo, and in one study of rats after Morris water maze training, caspase immunoreactivity was observed throughout the extent of hippocampal neurons and not just at synaptic sites (Dash et al., 2000). Third is the issue of containment; once caspase is activated, what prevents it from cascading forward to trigger cellular apoptosis?
Here we describe a series of studies designed to assess the role of caspase-3 activity in the phenomenon of song-specific habituation in adult zebra finches (Stoddard, 1996; Stripling et al., 2003; Stripling et al., submitted). This is an especially favorable model for analyzing biochemical changes associated with memory formation (Clayton, in press). A large discrete area in the forebrain mediates the representation of songs (Mello et al., 1992; Chew et al., 1996; Mello, 2002; Mello and Clayton, 1994; Mello et al., 1995; Phan et al., 2006; Stripling et al., 2001; Stripling et al., 1997). When a bird hears the same song repeatedly in the same context, the neurophysiological response to that specific song habituates; this habituation can persist for days (Chew et al., 1995; Chew et al., 1996; Stripling et al., 1997) or even longer (Phan et al., 2006). Song presentation also triggers robust molecular responses in this area, which also change as the presentation is repeated. Novel songs initially activate the ERK intracellular signaling pathway (Cheng and Clayton, 2004), followed by a pulse of zenk gene transcription (Kruse et al., 2000; Mello et al., 1992). When the stimulus is repeated across an hour or more, these molecular responses themselves habituate without affecting the responses to other songs (Cheng and Clayton, 2004; Kruse et al., 2004; Mello et al., 1995). Habituation of the zenk response to a song is correlated with emergence of a persistent change in the behavioral response to that song (Stripling et al., submitted; Stripling et al., 2003). The zenk gene response is especially easy to measure, and thus zenk gene expression in the auditory forebrain may be used as a molecular indicator of the status of a particular contextual song memory. If the song is heard as “novel” it induces a zenk response; after the song has been entrained it no longer induces zenk.
We show now that novel song exposure also triggers a rapid and transient increase in immunoreactivity for the activated form of caspase-3, and that caspase-3 activity is necessary for development of long-term habituation. The increase is specifically localized to postsynaptic terminals within the auditory forebrain, and we provide evidence for a molecular mechanism that could account for this tight temporal and anatomical control. These results establish a key role for caspase-3 in the machinery of memory consolidation.
Results
Caspase-3 response to novel song stimulation
Adult male zebra finches were placed in acoustic isolation and presented with a song stimulus the following afternoon. The stimulus (Methods) was a sequence of three different novel zebra finch songs repeated for 15 seconds followed by 45 seconds of silence, for a 1-minute total stimulation cycle. The cycle was repeated 2–90 times in different groups of birds. Immediately after the final stimulus, birds were euthanized and brains were collected and sectioned for immunocytochemistry (ICC). Our histological analyses focused on the caudomedial nidopallium (NCM), the part of the auditory forebrain that shows the most robust zenk gene response to song (Mello and Clayton, 1994). To probe for activated caspase-3, in initial experiments we used a primary antibody specific for the activated form and detected a significant increase in the auditory forebrain after song (not shown). We observed the same response but with lower background staining using the peptide DEVD (Fig. 1), which binds to the substrate recognition pocket of activated caspase-3 with high specificity; the DEVD itself was conjugated to biotin.
Figure 1. Increase and apparent synaptic association of active caspase-3 after novel song stimulation.
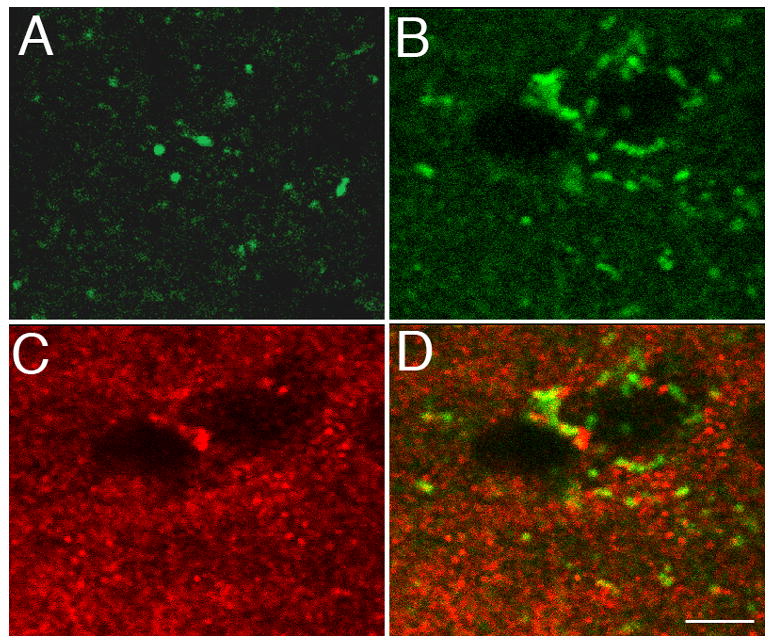
Shown are example confocal microscopic images of sections from a bird hearing only silence (panel A) or another bird immediately after 10 novel song presentations (B–D). The field of view is within the caudomedial nidopallium (NCM, in the auditory forebrain). The sections were double-labeled for active caspase (using biotinylated DEVD peptide detected by fluorescent-linked streptavidin, green fluor) and a synaptic marker protein, synaptotagmin (red fluor). Panel D: merger of panels B and C showing close association but distinct distributions of active caspase-3 and synaptotagmin immunoreactivities. Bar size = 4 microns.
DEVD binding in NCM was notably increased in song-stimulated relative to silent-control birds. When detected by DAB staining, the signal was diffuse and appeared to involve neuropil as well as cell bodies (not shown). When detected by fluorescent confocal microscopy, the signal was localized primarily to discrete puncta of various sizes that often outlined what appear to be unlabeled cell bodies or nuclei (Fig. 1). These puncta were present in the unstimulated control birds (Fig. 1A), but increased in frequency after song stimulation (Fig. 1B). A synaptic localization was suggested by double-label immunofluorescence using antibodies to the presynaptic marker synaptotagmin (Fig. 1C) or the postsynaptic marker PSD-90 (not shown). Both antibodies revealed puncta that are qualitatively similar and closely adjacent to the DEVD-labeled puncta, although more numerous and homogenous in their distribution (Fig. 1C, D).
To quantify the change in signal intensity following song stimulation, we calculated the fraction of pixels labeled above the background threshold in birds treated as matched pairs, one member of the pair hearing song and the other silence. We measured a mean increase of 3-fold in the NCM of birds sacrificed 10 minutes after song onset compared to their matched controls (p=0.002, paired t-test, n=7 pairs). No significant increase was detected in birds sacrificed at 2, 20, 30 or 90 minutes after onset of continuous song stimulation (Fig. 2). In examination of microscopic fields from other brain regions, we observed no significant effect of stimulus on labeling intensity in the overlying hippocampus, nor in any of the major telencephalic nuclei of the song control system (RA, HVC, lMAN, and Area X). Given the high rate of ongoing neuronal replacement in the auditory forebrain of adult zebra finches (Alvarez-Buylla and Nottebohm, 1988), we also considered the possibility that caspase activation would be associated with neuronal turnover. Using TUNEL staining, we looked for evidence of apoptotic cells at 6 and 24 hours after the stimulation used here but detected none (data not shown).
Figure 2. Time course of activated caspase-3 immunoreactivity in NCM during novel song playback.
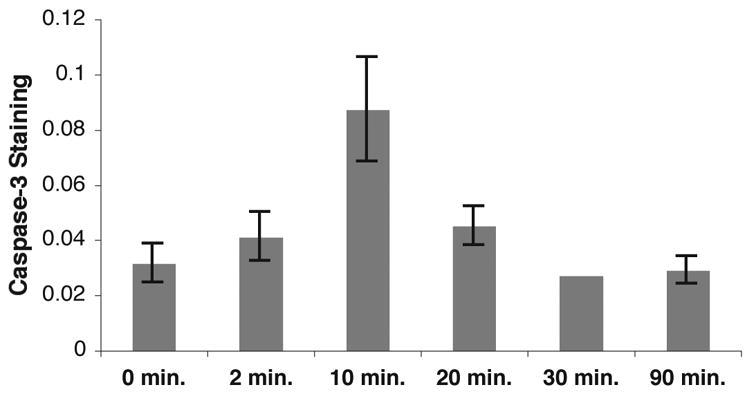
After overnight isolation in a sound chamber, each bird was presented with repeated playback of a song stimulus (15 seconds followed by 45 seconds of silence), and then euthanized at the time shown (relative to first stimulus onset). Brain sections containing NCM were probed with DEVD which was visualized by DAB staining using a 20x objective, and the signal above background was quantified (Methods). Numbers of birds at each timepoint (0, 2, 10, 20, 30 and 90 min, respectively): 7, 4, 8, 4, 3, 3.
To determine the cellular localization of the activated caspase with more precision, we employed immunogold electron microscopy, using avidin-gold to detect the bound biotinylated DEVD (Fig. 3). In a survey of 31 fields from control birds after silence, gold particles were observed in only 3 of the fields (9.7%), and in all cases they were located at or near postsynaptic densities inside the neuron (Fig. 3a). In sections from song-stimulated birds, however, gold particles were much more frequent (42 of 66, or 63% of all fields examined). In all cases the particles were in dendritic elements, and usually (35/42 positive fields) in spines identified by their post-synaptic densities and apposition to a vesicle-filled presynaptic terminal (Fig. 3b–3d). No gold particles were found in cell soma, nuclei, presynaptic terminals or surrounding glial projections.
Figure 3. Ultrastructural localization of activated caspase-3 by immunogold electron microscopy.
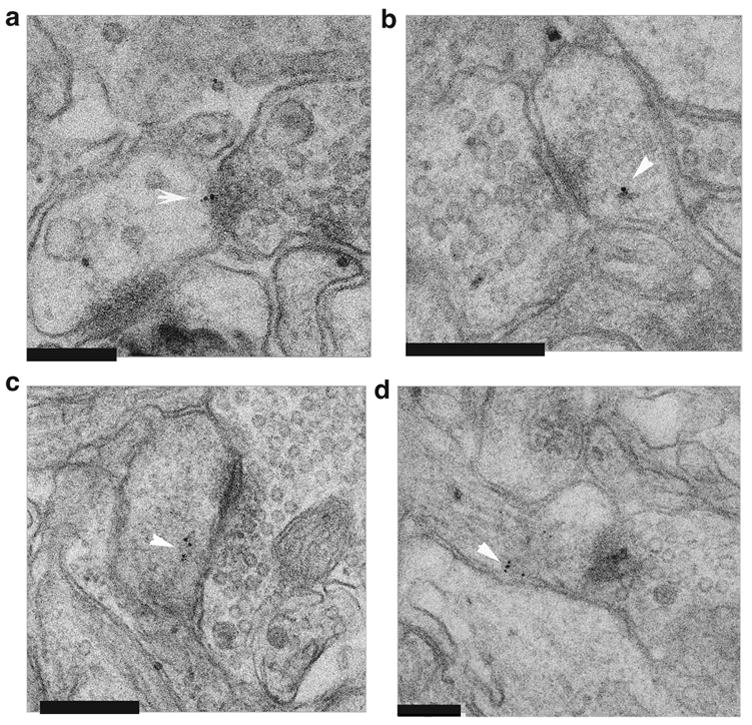
Representative images within NCM from birds after song or silence.
(A) showing one of the few [3/31, 9.7%] caspase-3 staining fields from silent control tissue.
(B–D) showing 3 of the more plentiful [42/66, 63%] staining fields from song stimulated tissue.
In each field, post-synaptic densities are evident with vesicle-filled presynaptic terminals on opposite side. Arrows note gold particles indicating caspase-3 location at the post-synaptic density (panel A) or nearby on the postsynaptic side (panels B–D). Size bars = 200 nm in all panels. No gold particles were found in soma, or presynaptic locations supporting a post-synaptic localization of this caspase-3 response.
Caspase-3 interactions with BIRC4
These results provide compelling evidence for rapid increases in activated caspase-3 protein at postsynaptic sites following song stimulation. Initially we assumed this change must involve de novo production of the active enzyme via cleavage of the proprotein. We attempted to measure a change in the amount of the cleaved active form of caspase-3 in brain homogenates using immunoblotting and immunoprecipitation (Fig. 4). Adult zebra finches were exposed to 10 min of song (Fig. 4, lanes 4 and 6) or silence (lanes 3 and 5), and their auditory lobules (AL) were dissected as in (Cheng and Clayton, 2004). In lanes 3 and 4, the extracts were immunoblotted with an antibody specific for the cleaved, active form of caspase-3. There was no detectable change in caspase-3 immunoreactivity following song stimulation in these or in three other replicate sets of samples. In lanes 5 and 6, the extracts were first enriched by immunoprecipitation using an antibody that recognizes both the activated form and uncleaved caspase-3 precursor, prior to immunoblotting with the antibody specific for the active form. Again, there was no detectable change in activated caspase immunoreactivity following song stimulation.
Figure 4. Caspase-3 is present in brain homogenates, but concentration does not change with song stimulation.
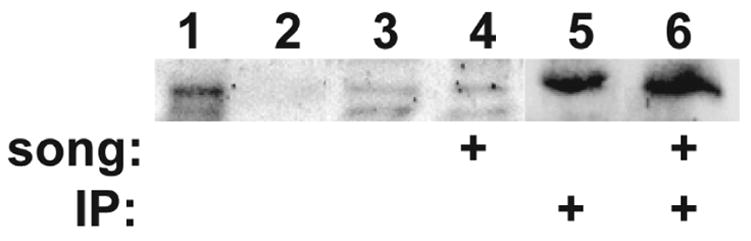
Auditory lobules (AL) were dissected from birds, homogenized, fractionated and immunoblotted. All lanes were blotted with anti-active caspase-3 antibody. Each lane represents the AL from a separate bird. The experiment was repeated a total of four times on 16 birds, and this figure is representative; quantitative densitometric analysis detected no significant effect of treatment. Activated caspase-3 often appears as multiple cleaved bands migrating variably in the 17–20 kD range (Jarskog et al., 2006; Faleiro et al., 1997); the doublet visible in lanes 3 and 4 aligns with the doublet indicated by arrows in Fig. 5B.
Lane 1, positive control showing human caspase-3 from transfected Jurkat cell line; Lane 2, negative control (non-transfected Jurkat cell homogenate); Lane 3, AL homogenate from a silent control bird; Lane 4, AL homogenate after 10 min. song stimulation; Lane 5, AL homogenate from a silent control bird after enrichment by immunoprecipitation with total caspase-3 antibody (both active and pro-form); Lane 6, as in lane 5 except AL homogenate was from a bird after 10 min. song stimulation.
Thus activated caspase-3 appears to be present in detergent-treated brain homogenates at a constant level independent of stimulation, even though probes for the active site detect local changes in fixed non-denatured tissue sections (Figs. 1–4). Moreover, we were unable to measure enzymatic activity for caspase-3 in homogenates of AL, before or after song or even after addition of a pharmacological activator, staurosporine. These results can be reconciled if activated caspase-3 is associated reversibly with an endogenous inhibitor. The amount of cleaved, activated caspase may not vary, but access to the active site of the enzyme may require release from this inhibitor. The process of homogenizing the tissue may effectively quench any active enzymes by exposing them to the inhibitor. There is in fact a known endogenous inhibitor of caspase-3, identified through in vitro binding studies of cloned proteins. Often referred to as xIAP (x-linked inhibitor of apoptosis protein), the inhibitor protein has been formally named BIRC4 by the HUGO Gene Nomenclature Committee (Wain et al., 2002; Wain et al., 2004). BIRC4 inhibits caspase by binding at its active site, the same site bound by both DEVD and the activated caspase-specific antiserum; this inhibition appears to be reversible (Huang et al., 2003; Takahashi et al., 1998).
To evaluate the possible association of activated caspase-3 with BIRC4, we first probed zebra finch brain extracts with an antibody to human BIRC4. We detected significant amounts of immunoreactive protein of appropriate size in extracts from various subregions of the zebra finch brain (Fig. 5A). Immunoprecipitation of zebra finch auditory forebrain extracts using the BIRC4 antibody co-precipitated activated caspase-3 (Fig. 5B). Conversely, co-precipitation of BIRC4 was also observed when anti-caspase-3 was the precipitating antibody (not shown). Confirming the specificity of the association, no caspase-3 immunoreactivity co-precipitated with tubulin, although the tubulin antibody brought down many other peptides evident by silver staining (not shown).
Figure 5. Association of caspase-3 and BIRC4 (xIAP) in brain homogenates.
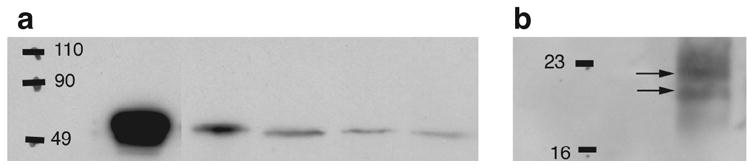
(A) Shows extracts of different regions of zebra finch brain immunoblotted with an antibody to BIRC4; lane 1: positive control peptide (human BIRC4); 2: cerebellum; 3: lateral forebrain; 4: medial forebrain; 5: auditory lobule of the forebrain (AL) which contains the song-responsive NCM.
(B) Co-immunoprecipitation assay, where an extract of AL was first immunoprecipitated with the BIRC4 (xIAP) antibody used in panel A, and then immunoblotted with the antibody to active caspase-3 as in Fig. 4. Arrows indicate the active caspase-3 doublet. This experiment was repeated four times using a different bird each time, with similar results.
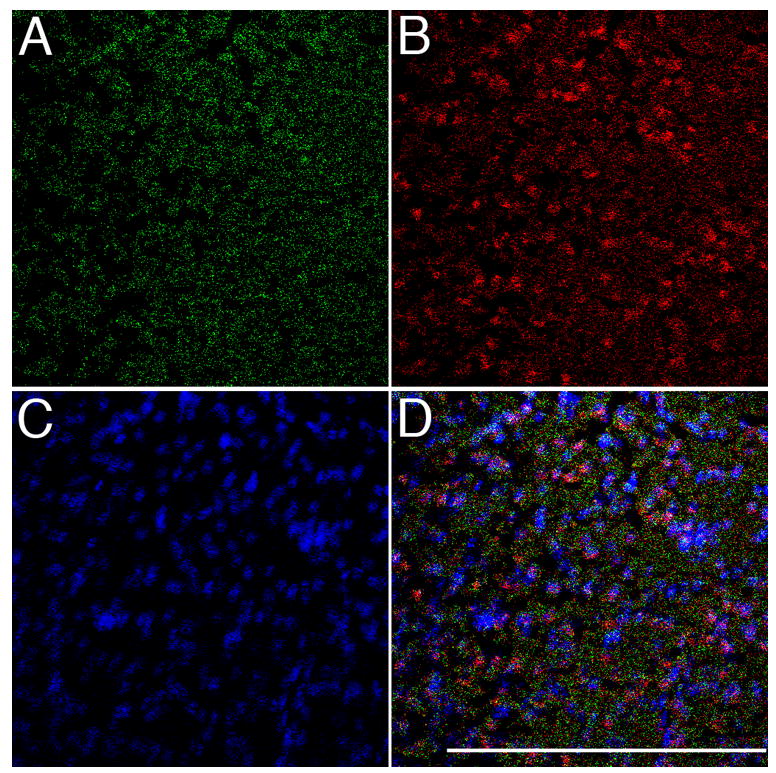
We then used multi-label immunofluorescence to assess the anatomical association of active caspase-3 and BIRC4, in birds after song stimulation. The BIRC4 antibody had been raised to a domain distinct from the caspase-binding site, so the BIRC4 signal should represent all the BIRC4 molecules. The DEVD signal should represent only activated caspase molecules unhindered by BIRC4 inhibition. DAPI staining was used to visualize cell nuclei, with the caveat that the treatments necessary for the immunochemistry result in spread of the DAPI signal outside of the nucleus and very weak labeling for some cells, especially large neurons. The results are illustrated in Figure 6. Active caspase (DEVD) again showed a very non-homogenous punctate distribution, with the puncta sometimes in neuropil but often clustered in patterns suggestive of cell shapes or boundaries (Fig. 6A). BIRC4 staining was closely adjacent, in some cases appearing to fill in the cellular shapes only suggested by DEVD puncta along the edges of the shapes (Fig. 6B, overlay in Fig. 6D). By careful inspection, weak DAPI staining could usually be seen associated with these shapes (Fig. 6C), consistent with a probable neuronal identify. The cellular elements with strongest DAPI staining (probable glia) were not typically associated with BIRC4 or DEVD labeling. Some sections in the same series were double-labeled with DEVD and an antibody for total caspase-3 (Fig. 7). Much like the BIRC4 antibody (red channel, Fig. 6), the total caspase-3 antibody (red channel, Fig. 7) generated strong cellular profiles that were only suggested by the puncta of DEVD labeling.
Figure 6. Colocalization of caspase-3 and BIRC4 (xIAP) immunoreactivity in NCM by double-label immunofluorescence microscopy.

Representative section of NCM from a bird hearing 10 min. of novel song, stained for: A) active caspase-3 using DEVD (green channel); B) BIRC4 (red channel); C) DAPI counterstain (blue channel); D) merged image of all three channels. Bar = 8 microns
Figure 7. Comparison of distributions for active caspase-3 (DEVD binding) and total caspase-3 (antibody immunoreactivity).
Representative section of NCM from a bird hearing 10 min. of novel song, stained for: A) active caspase-3 using DEVD (green channel); B) total caspase-3, both active and inactive forms (red channel); C) DAPI counterstain (blue channel); D) merged image of all three channels. Bar = 200 microns
These observations show that active caspase-3 is in close proximity to BIRC4 in NCM neurons, and are consistent with the hypothesis that caspase-3 activity can be regulated through interactions with BIRC4. In the context of the song habituation model, this hypothesis predicts that: 1) DEVD staining should increase after novel song; 2) DEVD staining should return to low levels as a song is habituated; 3) immunoreactivities for BIRC4 and for total caspase (active plus inactive forms) should remain constant during song stimulation and habituation. To test this, adult male zebra finches were placed in 3 groups and exposed to one of three treatments: Silence, Novel Song (10 minutes of playback), or Habituation (75 minutes of playback). Brains sections containing NCM were double-labeled using DEVD and antisera to either BIRC4 or total caspase (active and inactive forms combined). Mean intensities for each signal in each bird (fraction of pixels above threshold) were determined by confocal microscopy, and group averages of these means are presented in Figure 8. As in Fig. 2, song stimulation resulted in a large and specific increase in DEVD signal intensity relative to that in control birds hearing only silence. The fraction of pixels above background increased 9-fold and with habituation returned to the level of the silence control birds (p=0.03, ANOVA across the three treatments). Consistent with our proposed model of regulation, there was no significant change with stimulus condition in signal for either BIRC4 or total caspase (p>0.05, ANOVA across the three treatments for each probe). We do note the visible trends in the data suggesting possibilities that habituation might lead to a decrease in BIRC4 signal, and that both song treatments might lead to a decrease in total caspase signal. These trends did not achieve statistical significance in this data set but may be worth examining further in future studies.
Figure 8. Effects of song stimulation and habituation on caspase-3 and BIRC4 immunoreactivities in NCM.
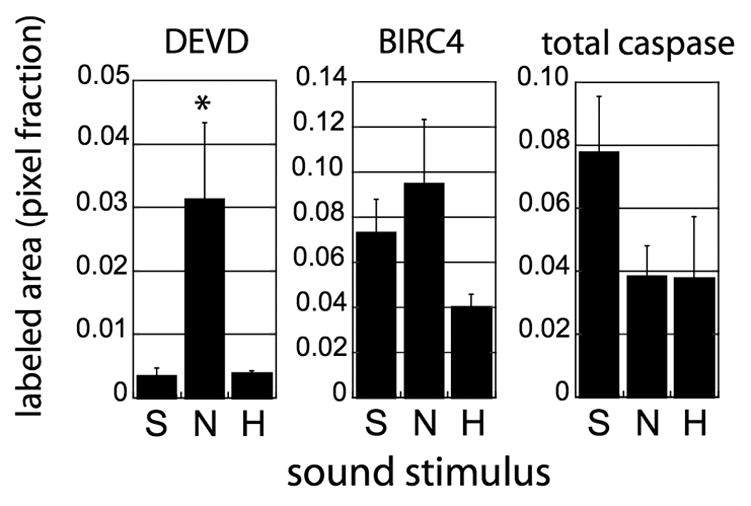
Adult male zebra finches were placed in 3 groups treatment groups (n=4 per group) based on the sound stimulus to which they were exposed immediately prior to euthanasia: S, silence; N, novel song (10 minute playback); H, habituation (75 minutes of playback). Initial stimulations were performed simultaneously on 4 birds per day (with counterbalancing of treatment groups across 3 days), and subsequent manipulations were performed in parallel on the tissue from all 12 birds. The brains were sectioned and double-labeled as in Figs. 6 and 7, in an alternating sequence using DEVD and antisera to either BIRC4 or total caspase (active and inactive forms combined), with DAPI as a counterstain. Images were collected from 4–7 fields within NCM of each section, and the red and green channels of each image were analyzed separately as in Methods to generate a mean fraction of pixels above background threshold for each probe in each bird. The averages of these means for each treatment group are presented in the graph. Bars indicate standard error, and the asterisk indicates a significant effect of treatment on that measurement (ANOVA comparing the S, N and H treatment groups for each probe).
Involvement of caspase-3 in persistent song habituation
The timecourse of active caspase-3 release following novel song exposure (Fig. 2) is delayed relative to the onset of short-term spike response habituation (within seconds, (Chew et al., 1995; Stripling et al., 1997)) or ERK phosphorylation (< 2 minutes, (Cheng and Clayton, 2004)). Other observations suggest that events during the 30–60 minutes following onset of song training are critical to establish a song memory that will persist for at least a day and manifest itself as a discrimination in zenk, behavioral and neurophysiological responses (Chew et al., 1996; Stripling et al., submitted). Might caspase-3 release have some role in establishment of persistent (long-term) habituation?
As a direct test of this in the adult zebra finch model, we asked whether pharmacological inhibition of caspase-3 activity in NCM during song training would disrupt the development of a zenk habituation memory. Birds were trained by repetition of one song stimulus for 75 minutes as in Figure 2, and then tested one day later for the memory of the training song using the zenk assay (Fig. 9A). Thirty minutes prior to onset of training, a cell-permeable caspase-3 inhibitor was infused via cannula into NCM; we used a dosage sufficient to inhibit caspase-3 activity for at least 3–4 hours following intrathecal administration in rats (Springer et al., 1999). When these birds were tested a day later by presentation of the same song, a full zenk response was observed, as though the song were novel to them (Fig. 9B, left). Birds that received the same cannula treatment but were infused with only vehicle during training showed the diminished zenk response indicative of habituation to that song (Fig 9B, right). The difference in zenk responses between the inhibitor and vehicles treated groups was significant (Fig. 9C).
Figure 9. Requirement of caspase-3 activity for long-term zenk habituation.
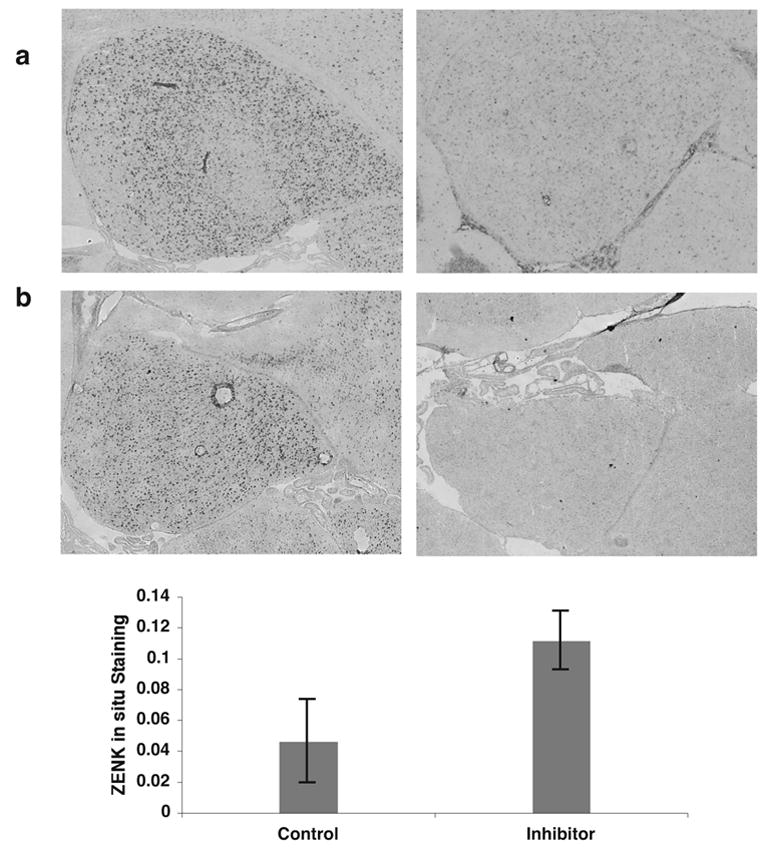
A) Reference sections showing in situ hybridization for zenk mRNA in NCM, in birds hearing 30 min. of either (left) novel or (right) familiar song. The lack of zenk response to familiar song indicates the presence of a song-specific memory (Kruse et al., 2004; Mello et al., 1995; Stripling et al., submitted).
(B) Example in situ hybridizations showing effect of caspase-3 inhibition during training on the appearance of a song memory (persistent zenk habituation) a day later. Birds were trained on day 1 and tested with the same song on day 2. Left: caspase-3 inhibitor had been infused during training; note zenk response similar to bird hearing novel song (as in panel A, left). Right: vehicle (only) had been infused during training; note zenk response similar to bird hearing familiar song (as panel A, right). Images in A and B are tiled 6x5 grids of 10x microscope field views to visualize the whole of NCM.
(C) Quantification of zenk in situ hybridization data from four trials of paired injections (n=8 birds total) performed as the example in panel B. Mean zenk hybridization intensity for each treatment group is expressed as a fractional area of labeling as in Figs. 2 and 8 (Methods). Each trial involved two birds treated in parallel, one receiving the inhibitor and the other only vehicle during training, and then tested a day later for their zenk responses to the training stimulus. The low level of zenk in the controls indicates persisting habituation to the stimulus. Birds that had been infused with inhibitor during training showed a significantly higher zenk response at test (p=0.01, two tailed paired t-test), indicating disruption of long-term zenk habituation by drug treatment during training. Error bars: S.E.M.
As an additional test of a link between caspase-3 activity and persistent habituation, we asked whether habituation of the caspase-3 response itself can persist for a day. On the first day, pairs of birds were trained by repetition of the same song stimulus for 75 minutes. On the following day, the birds were tested with 10 repetitions of song, one member of each pair hearing the training song and the other hearing a new one. The birds were immediately euthanized and active caspase-3 levels in NCM were assayed using immunohistochemistry for DEVD binding as in Figure 2. The birds tested with the training song showed a greatly reduced level of active caspase-3, three-fold less than in the birds tested with novel song (p=0.003, two tailed t test of paired means, n=6 pairs), indicative of caspase-3 habituation to that song. Thus caspase-3 activity is necessary for the development of long-term habituation to a song, and a consequence of that habituation is a stimulus-specific change in the caspase-3 response itself.
Discussion
The term “synapoptosis” has been coined to suggest the possibility that apoptotic processes are redirected in neurons to regulate synaptic turnover (Mattson and Duan, 1999; Mattson et al., 1998). Although the idea is attractive, direct experimental support has been lacking. Changes in caspase activity have not been detected in any animal model of learning and memory, no evidence has been shown for specific synaptic localization of active caspase in the brain, and no mechanism has been proposed for how caspase activity might be confined or constrained to prevent catastrophic cell death.
Our results now address each of these three missing points. First, we observed that the density of caspase-3 active sites increases transiently in songbird auditory forebrain during song habituation training, an emerging model for study of biochemical and molecular mechanisms of memory. Pharmacological interference with caspase-3 during training blocked the appearance of a persisting memory evident a day later in the habituated zenk response. Second, by immunoelectron microscopy, we detected the change in active caspase-3 specifically in postsynaptic terminals of the auditory forebrain. Finally, we found that activated caspase-3 is present even in unstimulated brain but bound to an endogenous inhibitor, BIRC4. The amounts of BIRC4 and total caspase immunoreactivity do not change significantly with song stimulation or habituation, despite the rise and fall in active caspase-3. These results lead to a model in which active caspase-3 is always present in synaptic terminals but sequestered, and released only transiently to effect a synaptic process essential for memory storage.
Our observations hinge on accurate detection of the activated form of caspase-3 in the zebra finch brain. A caspase-3 ortholog has been formally identified in chicken and ESTs representing the presumed ortholog are present in cDNA libraries prepared from zebra finch brain (Experimental Procedures). We specifically detected the activated form of the protein using two different types of reagent. For analysis of extracts by immunoprecipitation and immunoblotting, we used two different antibodies that recognize the epitope formed upon cleavage of the proprotein. These antibodies have been used to detect active caspase-3 in mouse hippocampus (Dash et al., 2000) and in cultured hippocampal neurons (Mattson et al., 1998). For analysis of tissue sections, however, we obtained cleaner results using the peptide DEVD, which binds specifically to the tertiary active pocket formed by the assembly of the four subunits of the active enzyme. In mammals, DEVD also binds though with lower affinity to caspase-7 (Chan and Mattson, 1999); no caspase-7 ortholog has been identified in the current draft of the chicken genome (http://www.chick.umist.ac.uk/; http://genome.ucsc.edu; http://www.tigr.org) or the zebra finch brain EST collection (http://titan.biotec.uiuc.edu/songbird). The lack of candidate cross-reacting proteins, the appropriate target size on immunoblots, and the consistent detection of the same apparent target using both antibody and peptide binding give confidence that the target under study here is indeed the zebra finch caspase-3 protein.
With the DEVD method, we observed a significant increase in binding to brain sections within 10 minutes following onset of novel birdsong and a return to baseline by 20 minutes; subsequent exposure to the same “familiar” song no longer induced this change. We also detected an increase using immunohistochemistry with antibody to the active protein (not shown), although levels of non-specific background staining were higher with the antibody than with DEVD. This represents the first direct evidence of a change in availability of the activated caspase-3 protein in the brain during the process of memory formation. Dash et al (2000) also detected the activated protein in the rodent hippocampus, but were unable to measure any change by antibody staining in rodent hippocampus 15 minutes after onset of Morris water maze training. It would be interesting to test for changes in activated caspase-3 at earlier time points in water maze training.
The results here indicate that the brain’s caspase response to novel experience is surprisingly fast, especially compared to the apoptotic activation of caspase-3 which typically develops over a period of 30 minutes to 24 hours (Glazner et al., 2000; Keane et al., 1997; Mattson et al., 1998; Zhuang et al., 1999). The timecourse is similar if not even delayed, however, relative to that observed for various other physiological responses to novel birdsong. ERK phosphorylation peaks about 2 minutes after stimulus onset (Cheng and Clayton, 2004) and single-unit spike responses are modulated downward over about the same duration (Stripling et al., 2001; Stripling et al., 1997). The zenk response to a novel song is initiated by just a few seconds of song exposure (Kruse et al., 2000), although the process then continues over the next 1–2 hours through the rise and fall of zenk mRNA and then protein (Mello and Ribeiro, 1998; Mello and Clayton, 1994; Mello et al., 1995). We considered whether caspase activity might be necessary for induction of the zenk mRNA response itself, but infusion of DEVD prior to song stimulation had no apparent effect on this (data not shown).
Behavioral studies in rats have demonstrated effects of various caspase inhibitors in assays of learning and memory (Bilbo et al., 2005; Dash et al., 2000; Gemma et al., 2005; Stepanichev et al., 2005) but there is some disagreement about the exact timing of the behavioral effect. Dash et al (2000) observed that infusions of a broad-spectrum caspase inhibitor (BAF) into the hippocampus immediately after training in the water maze had no initial effect on retention in animals when tested 30 minutes later, but disrupted memories when tested 24–48 hours later. From this they concluded that caspase-3 activity is necessary for long-term memory consolidation but not short-term memory. However, the rapid and transient timecourse of the caspase-3 response we observed (peaking 10 minutes after onset of training) suggests that infusion even immediately after behavioral training might still fail to block the peak of the primary response. In contrast, Stepanichev et al. (2005) infused the peptide DEVD into the lateral ventricles of rats, waited a day before training in an active avoidance paradigm, and found a significant effect at that point on the initial rate of learning. To reconcile this with the results of Dash et al. (2000) and their own prior studies of long-term potentiation in hippocampal slices (Kudryashov et al., 2004), they speculated that the learning effect may have been due to loss of a caspase substrate and not caspase itself
Consideration of our inhibitor experiment (Fig. 9) along with our timecourse analyses (Fig. 2) suggests that caspase-3 activity is most likely involved at an early stage in the consolidation of initial (short-term) memories into persistent (long-term) ones. The concept of phasic consolidation runs deep in the literature of learning and memory research, (e.g., (Millin et al., 2001; Bailey et al., 1996; McGaugh, 2000; Dudai, 2004; Guzowski, 2002). It would be interesting to probe the effects of caspase-3 inhibition on habituation of spike responses during song training (Stripling et al., 1997; Chew et al., 1995), which begins almost immediately upon stimulus repetition (“short-term habituation” or response modulation) but consolidates into a persistent memory only after 30–60 minutes. From the present results, we predict that blocking caspase-3 activity will have little effect on the initial downward modulation of spikes, but should inhibit the emergence of persistent (long-term) spike habituation. A possible mechanism for prolonging or augmenting spike habituation is suggested by evidence that microinjected caspase-3 will cleave AMPA receptors and thereby reduce glutamatergic responses (Lu et al., 2002).
It would also be desirable to develop a behavioral assay in songbirds for effects of caspase inhibition on memory formation, to complement the zenk assay we used here. We were unable to use the song listening response assay (Stripling et al., submitted; Stripling et al., 2003), as we have found in other work that the behavioral “listening” response to novel song is disturbed by cannula manipulations (Dong and Clayton, ms. in prep.). Another opportunity for future behavioral studies would be to test for effects of caspase inhibition during juvenile song training, when a male is learning to copy the song of a tutor (Tchernichovski et al., 2001).
Our study provides the first direct evidence not only of caspase-3 activation by animal training, but also of localization to post-synaptic terminals in the brain. Reagents specific for the activated enzyme (either DEVD or antisera) reacted specifically with synaptic terminals as indicated by both double-label fluorescence microscopy (Fig. 1) and electron microscopy (Fig. 3). Moreover, double label confocal analysis suggested a more restricted distribution for activated caspase-3 than for synaptotagmin (Fig. 1) or PSD-95 (not shown), suggesting that active caspase–3 may be released in only a small subset of synapses by a particular stimulus. The EM analysis suggests that song stimulation not only increases the amount of accessible active sites but also changes their subcellular distribution, as they appear to be farther from the PSD. This could indicate either a physical transport of the protease after activation or an unmasking of latent activated caspase-3 at sites removed from the PSD. This observation merits further study with more samples and additional timepoints, as it may provide clues to the subcellular and temporal organization of synaptic remodeling.
Despite detecting the activated caspase-3 protein in tissue sections using two different reagents, we were unable to measure any enzyme activity in brain extracts using a standard biochemical assay. Nor were we able to see a stimulus-associated increase in the amount of the cleaved active protein by immunoblotting. To account for these differences, we hypothesized that caspase-3 is associated in brain homogenates with the inhibitor BIRC4. The presumed ortholog of BIRC4 is present in the zebra finch brain EST library (Experimental Procedures). Consistent with our hypothesis, we found that BIRC4 co-precipitates with activated caspase-3, and vice versa. We did not observe co-precipitation of either caspase-3 or BIRC4 when an abundant control protein, tubulin, was precipitated (not shown). By confocal microscopy and immunolabeling, we showed that BIRC4 is co-localized with activated caspase protein in fixed tissue sections. The most parsimonious interpretation is that a pool of caspase-3 is maintained in the cleaved, active state in the brain, and released and quickly re-sequestered in a complex with the inhibitor, BIRC4.
Extrapolating from studies of apoptosis, interactions between caspase-3 and BIRC4 in synaptic terminals may be sensitive to intracellular signals of electrical activity such as an increase in synaptic Ca++ (Boehning et al., 2003; Mattson and Chan, 2003) or activation of kinase/phosphatases, and may involve interaction with other regulatory proteins such as the mitochondrial proteins Smac/DIABLO (Korhonen et al., 2004; Saito et al., 2003). Indeed, we detected several other peptides in the co-precipitates (not shown), and are currently applying proteomic methods to identify these other proteins. We hypothesize that these proteins comprise a caspase-3 regulatory assembly specific for postsynaptic terminals, and suggest the term “plastisome” to describe this assembly.
Caspase-3 has many potential degradative targets in the synapse including structural and signaling proteins such as actin, AII/BII spectrin, vimentin, focal adhesion kinase, gelsolin, and AMPA glutamate receptors (Chan and Mattson, 1999; Wang, 2000). Others have speculated how a brief, localized wave of targeted proteolysis might contribute to various phenomena in synaptic plasticity, such as synaptic depression, synaptic tagging, or control of synaptic silencing (Frey and Morris, 1998; Lugli et al., 2005). Our results establish that caspase-3 likely plays a key role in these processes, independent of its role in apoptosis. Thus, from cell nucleus (Clayton, 2000) to synapse, neurons appear to have modified the general machinery of cell growth control to mediate their requirements for lifelong plasticity.
Experimental Procedures
Assessment of Orthology
Existence of orthologous sequences and expression in zebra finch brain was assessed formally by analysis of the Songbird Neurogenomics Initiative zebra finch brain EST collection (ESTIMA, http://titan.biotec.uiuc.edu/songbird/). For caspase-3, two zebra finch ESTs (Genbank accession numbers CK310167 and CK314899) form a contig (PTA_05.5588.C1.Contig6178) of 774 nucleotides, which is annotated as caspase-3 based on overlap of 182 predicted amino acids at 84% identity (e−106 by tblastx against TIGR Gallus gallus EST) against chicken caspase-3. The chicken caspase-3 sequence (Unigene ID Gga.4346, Gallus gallus LOC395476, 283 amino acids) shares 66–77% identity with various mammalian caspase-3 isolates; in comparison the zebra finch EST-derived fragment is 72% identical the human caspase-3 precursor. For BIRC4, zebra finch EST SB03005B2E12.f1 (Genbank accession number DV946631) is 77% identical (e−120) to the chicken ortholog over 230 predicted amino acids, and two other non-contiguous ESTs cover both the n- and c-termini of the protein with similar levels of local identity. The chicken BIRC4 ortholog (UniGene Gga.104, UniProt Q8UVF8, 493 amino acids) is 57% identical to the human protein of 492 amino acids, whereas the predicted zebra finch protein is ~50% identical to human.
Antisera and Peptides
For all immunomicroscopy and immunoblot analyses of caspase, experiments were performed and similar results were obtained using commercial antibodies from two different sources, raised against the human protein. Antibodies that recognize total (uncleaved and heavy chain) caspase-3 were from Pharmingen and Research Diagnostics. Antibodies specific for the cleaved, activated caspase-3 were from Cell Signaling Technologies and Pharmingen. Active caspase was also detected using the modified peptide DEVD-CHO, conjugated to biotin (BIOMOL Research Laboratories, Inc). Other antibodies used included monoclonal anti-neurofilament 145kDa (Oncogene), monoclonal anti-synaptotagmin (Chemicon International Inc.), monoclonal anti-postsynaptic density protein 95 (Chemicon International Inc.), anti-neuron specific enolase (Biogenesis Inc.), anti-glial fibrillary acidic protein (Chemicon Inc.), monoclonal anti-human-XIAP/BIRC4 (Chemicon International Inc.) and monoclonal anti-chicken-alpha-tubulin (Sigma).
Animals, song stimulation and tissue collection
Male zebra finches were acquired from a commercial zebra finch supplier (Magnolia Bird Farms, Pasadena, CA) at 60–90 days of age and housed three males per cage in our colony at the Beckman Institute (University of Illinois) to full adulthood (120–200). To assess responses to novel song, the afternoon before stimulation two birds were transferred to adjacent acoustic isolation chambers (Tracor, Inc.). The following afternoon, after the birds were quiet for at least 1 hour, one bird was exposed to song playback and the other to continued silence. The song stimuli were digitized recordings from an archive made previously at Ohio State University (Dr. Susan Volman). Each song stimulus comprised three different five-second zebra finch songs played back to back, structured as a typical bout of singing. The same stimulus was presented once per minute (i.e., 15 seconds of song and 45 seconds of silence) using Syrinx software (University of Washington, Seattle) over the period indicated in each figure legend. This presentation schedule was chosen as it had been previously shown to induce a robust zenk IEG response (Mello and Clayton, 1994). As soon as song playback was initiated in one chamber, the matching silent control bird was euthanized and the brain collected. Immediately following the last song presentation, the stimulated bird's brain was then collected. To assess persistence of song habituation, the same basic procedure was used except both birds were exposed to repetitions of one stimulus for 75 minutes, then kept in the isolation chambers for an additional 24 hours. They were then exposed to 10 min of either the same stimulus or a different stimulus prior to euthanasia. The stimulus design for both exposures was the same as described above (i.e., each stimulus repetition was a sequence of 3 songs over 15 seconds followed by 45 sec of silence). Birds were euthanized by decapitation, brains were rapidly dissected and the whole brain and cerebellum were removed and submerged in a brain mold containing Tissue Tek (VWR), a cryoprotectant compound. The brain mold was placed in a dry ice ethanol bath and allowed to freeze. From capture to frozen this process took about three minutes.
Immuno-Light Microscopy
Parasagittal sections were cut by cryostat (20 micron thickness for DAB staining, 12 microns thick for immunofluorescence), thaw mounted, fixed in 3% paraformaldehyde for 5 minutes, dehydrated, air dried, and stored at −80°C until use. Selected slides containing NCM were blocked with avidin and goat serum, washed and exposed to antisera (overnight at 4°C, followed by secondary antibody) or DEVD-CHO conjugated to biotin (48 hours at 4°C). For double-immunofluorescent staining, tissue was first stained for DEVD-biotin as above. After DEVD-biotin washout, avidin-fluor Alexa-fluor 488 (Molecular probes, green fluor) was applied for 1 hour, followed by avidin blocking again and then one of the primary antibodies. Biotin conjugated secondary antibody and then streptavidin linked to Alexa-fluor 564 (Molecular Probes Inc) were applied. The tissue was washed and stained with DAPI (15 min) and mounted in Fluoro Guard Antifade Reagent (BIO-RAD, Inc.) and stored in the dark at 4°C. Control slides received no DEVD or primary antibodies but received all secondaries and fluors in the same order as each group of experimental slides. For DAB staining (of sections at 20 microns, Figs. 2 and 8), after application of primary and secondary antibodies, the sections were rinsed and exposed to Vector Labs ABC solution for 30 minutes. The tissue was rinsed, exposed to freshly prepared DAB (Sigma Fast DAB Kit) for exactly 2 min. The reaction was stopped by immersion in ddH2O. The tissue was mounted into crystal mount (Fisher), dried, and visually inspected the following day.
Fluorescent samples were visualized at the Beckman Institutes Image Technology Group Microscopy suite using a 63x objective, Zeiss confocal microscope, associated software (Zeiss), and an Airy 1 pinhole which yields a depth of field (optical thickness) of approximately 0.5 microns. The exciting lasers were carefully calibrated to eliminate cross excitation of the fluors. For the quantitative analysis in Fig. 8, background threshold was set using the matching control slide for each labeling sequence and the laser intensity was adjusted manually until this slide appeared black; all experimental slides from the same labeling sequence were then processed at the same setting. Images were converted to grayscale and inverted and imported into MCID software program (Microcomputer Imaging Device Software system, Imaging Research Inc.). Isolated single pixels were ignored (i.e., minimum target size criterion: two adjacent pixels) and the proportional area (pixel fraction) showing signal above the background threshold was calculated. Similar results were obtained by measuring the entire field at low confocal magnification (as in Fig. 7), or by sampling random BIRC4-labeled profiles at higher confocal magnification (16x16 microns, similar to Fig. 6). Quantitative analyses of DAB stained sections were performed on tiled, digitized grayscale images constructed from fields collected using a 20x objective. The threshold for significant signal intensity was set for each image to be equal to the 95th percentile of pixel intensities within the cerebellar white matter on that section, and isolated single pixels were ignored (i.e., minimum target size criterion: two adjacent pixels). Within each NCM, the proportional labeled area (number of pixels above threshold divided by total number of pixels) was determined using MCID software. Statistical analysis was by two-tailed t-test for paired means, where the pairs were sections from matching experimental and control birds that had been stimulated, euthanized and processed in parallel.
A TUNEL assay (TdT-mediated dUTP nick end labeling), for chromatin condensation associated with apoptotic cell death, was performed with a kit purchased from Roche.
Immuno-Electron Microscopy
Tissue was collected from song-stimulated brains and immediately immersed in ice cold Karnovsky’s fix (2%glu, 2.5% paraformaldehyde) in 0.2 M Sorenson’s buffer (28ml 0.2M NaH2PO4, 72ml 0.2M Na2HPO4, ph 7.2) prepared fresh from EM grade glutaraldehyde and paraformaldehyde stock, for 75 min. The tissue was moved to 1% paraformaldehyde in 0.2M Sorenson’s for an additional 4 hours at 4°C. Tissue was rinsed in Sorenson’s 3x45 min., and immersed in Sorenson’s with 50nM biotin-DEVD-cho (BioMol) for 48 hours. Tissue was rinsed as above, and immersed in 1:100 streptavidin-gold (12nm) for 48 hours. The tissue was rinsed and placed in 2% Osmium (OsO4) in 0.2M Sorenson’s final buffered concentration (1:1 4% osmium to 0.4M Sorenson’s) for 1 hour and rinsed 3x 10 min in Sorenson’s. The tissue was then placed in ice-cold 0.1N sodium acetate for 3x2 min washes and then into 0.5% uranyl acetate for 30 min in the dark (the uranyl acetate was syringe filtered just prior to use), and then rinsed 3x2 min in 0.1N sodium acetate on ice. The tissue was then dehydrated on ice, 5 min each step of 30%, 50%, 70%, 80%, 95% ethanol and then 3x10 min in fresh 100% EtOH. The tissue was then placed in propylene oxide 2x15 min and allowed to come to room temperature in these washes. The tissue was then placed in 1:1 propylene oxide:resin mixture in glass vials with rotation overnight (8 hours). Resin mixture was 16ml Embed-812 (Epon-812 substitute), 8.6ml NMA, 11.2 ml DDSA. This mix was used for all infusing steps, and then when hardening was performed (below) 0.64ml of DMP-30 was added to the resin mix. The tissue was then immersed in 1:3 propylene oxide and resin mix for 8 hrs at room temperature with rotation, and then in 100% resin mix overnight with rotation. Finally the tissue was placed in rubber block molds with paper/pencil (or Helvetica 6 point font) labels and immersed in hardening resin mix as described above, and baked at 60°C for 48 hr. The blocks were allowed to cool, checked for hardening, and stored until sectioning.
Immuno- and Enzyme Assays on Homogenates
Following stimulation (10min novel song as above) the bird was decapitated, the skull was bisected down the midline, and the exposed NCM-containing auditory lobule (AL) was collected with a hippocampal knife and placed in homogenization buffer: 50mM Pipes/NaOH (pH 6.5), 2 mM EDTA, 0.1% Chaps, 5 mM DTT. For immunoblot analyses, the buffer also included 20 μg/ml Leupeptin, 10 μg/ml pepstatin, 10 μg/ml aproptinin and 1 mM PMSF, 3% Triton X-100, and 50 nM DEVD to block all activity of the enzyme in the homogenate. Protein concentration was determined for each sample and equivalent amounts were added to each lane of an SDS-PAGE, run for 1 hour, electroblotted to a nylon membrane, blocked, and exposed to antibodies as indicated in the text. Secondary antibody was applied (1 hour room temperature) and if necessary ABC amplification (Vector Labs) of the signal was performed. Quantitative analyses were performed using NIH Image software.
Immunoprecipitations were performed using the homogenization buffer but no additives, which is optimized for maintaining native structure and enzymatic activity. The tissue was placed in 100–200 μl of buffer and homogenized with a dounce and motor assembly in the microcentrifuge tube for 1 min. The homogenates were centrifuged at 14,000 rpm for 10 minutes. The supernatant was transferred to a fresh tube and stored on ice while a small fraction was assayed for total protein concentration. The precipitating antibody was linked to a protein A coated plastic bead, added to the supernatant, and incubated with end-over-end rotation at 4 C overnight. Beads were collected by centrifugation, washed, boiled in SDS loading buffer for 4 min, and the supernatant from these tubes was loaded onto SDS-PAGE gels.
Attempts were made to measure caspase-3 activity in homogenates of AL using the Caspase-3 Cellular Activity Assay kit from Biomol (Plymouth Meeting, PA) which is based on a fluorometric caspase-3 substrate, with provided enzyme as a positive control and staurosporine as an exogenous activator.
Infusion of caspase-3 inhibitor
Birds were initially implanted with a cannula as follows. Birds were anesthetized with 3-4 ml/kg of a pentobarbital/chloral hydrate cocktail similar in composition to Equithesin (2.12% w/v MgSO4, 10% v/v ethanol, 39.1% v/v propylene glycol, 0.98% w/v sodium pentobarbitone, 4.2% w/v chloral hydrate), restrained with cloth jacket and in a stereotaxis apparatus (H. Adams, Caltech Central Engineering) with the horizontal head axis 35° relative to the vertical axis of the instrument. A small circle of the skull was removed and an opening was cut in dura 50 microns lateral and 70 microns anterior to Y0, centered over the region of the greatest zenk response to song (Mello and Clayton, 1994) and a pre-cut (2 mm length) 26G guide cannula (Plastic One, VA) was implanted. A 33G dummy cannula was inserted to prevent tissue clog. The cannulae were fixed at the skull with dental cement (Grip Cement, L. Caulk Co., IN). Following surgery, each bird was individually isolated in an acoustic-attenuation chamber overnight for recovery, then was transferred back to animal quarters for 6–7 days before the drug injection was performed. The afternoon before microinjection, the bird was transferred to acoustic-attenuation chamber for overnight isolation. Immediately before injection, the dummy cannula was removed from the guide cannula and replaced with a 33G internal cannula, which was connected via PE20 tubing to a Hamilton microsyringe driven by a microinfusion pump (kdScientific, New Hope, PA). Microinjection was performed unilaterally in a 1 μl volume delivered over 2 min. The injection cannula was left in position before withdrawal for an additional 1 min to minimize dragging of the injected liquid along the injection tract. The cell permeable and specific inhibitor had the following sequence: N-acetyl-Ala-Ala-Val-Ala-Leu-Leu-Pro-Ala-Val-Leu-Leu-Ala-Leu-Leu-Ala-Pro-Asp-Glu-Val-Asp-cho). This represents DEVD-cho with an n-terminal linkage to the hydrophobic region of the signal peptide of Kaposi fibroblast growth factor (Biomol Research Lab. Inc.), which has been shown to enhance the cell permeability of peptides. The inhibitor DEVD-cho is highly specific to caspase-3 (Ki<1nM) and reversible. Inhibitor was solubilized in 50% DMSO/50% saline to 3 nmol of peptide per μl, to give a dosage in the range established for sustained caspase inhibition over several hours in rodents (Springer et al., 1999; Dash et al., 2000). The bird was released into a cage with food and water, allowed to rest for 1 hour, then trained with 75 minutes of song stimulation as above. The bird remained in the chamber over night and the following day either the same song was presented for 30 minutes and the brain was immediately collected and frozen for analysis of zenk mRNA expression by in situ hybridization.
zenk in situ Hybridization
The hybridization has been described in detail (Kruse et al., 2000). Briefly, parasagittal sections were cut on a cryostat, dehydrated and stored frozen. Sections were then rehydrated, antisense zenk probe in hybridization solution was prepared and added, and the slide was incubated under mineral oil for 3hr at 65°c. The oil and coverslips were removed followed by three stringent washes at 65°c, two of 2X SSPE and 50% formamide, and one of 0.1X SSPE. The slices were then placed in a humidified chamber and blocked overnight at 4°c. The following day, they were washed, incubated with horseradish peroxidase linked Fab anti-DIG antibody fragment (Boehringer Mannheim) for 2 hours at room temperature, and washed again. A color detection reagent solution of BCIP/NBT (Sigma fast tablets) was placed on each slide. Slides were incubated at room temperature from two hours to two days depending on the probe and probe concentration. Developed slides were mounted in crystal mount (Fisher). Analysis of the zenk staining followed the methods used in the analysis of the caspase-3 DAB stains, except that the hippocampal area was used as the control for thresholding instead of cerebellar white matter. The size range of positive targets for counting was between 30um2 and 250um2.
Acknowledgments
Supported by NIH grants R01 MH52086, R01 NS051820 and RO1 NS045264 (to DFC), and NRSA 1 F30 NS45379-01A2. Thanks to Alex Dirlam, Annie J. Kannankeril, and the William T. Greenough lab for technical assistance, to Scott Robinson of the Beckman ITG for advice and assistance with electron microscopy, and to Julia George for assistance with interpretation and presentation of confocal data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afford S, Randhawa S. Apoptosis. Mol Pathol. 2000;53:55–63. doi: 10.1136/mp.53.2.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Nottebohm F. Migration of young neurons in adult avian brain. Nature. 1988;335:353–354. doi: 10.1038/335353a0. [DOI] [PubMed] [Google Scholar]
- Bailey CH, Bartsch D, Kandel ER. Toward a molecular definition of long-term memory storage. Proc Natl Acad Sci USA. 1996;93:13445–13452. doi: 10.1073/pnas.93.24.13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Biedenkapp JC, Der-Avakian A, Watkins LR, Rudy JW, Maier SF. Neonatal infection-induced memory impairment after lipopolysaccharide in adulthood is prevented via caspase-1 inhibition. J Neurosci. 2005;25:8000–8009. doi: 10.1523/JNEUROSCI.1748-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehning D, Patterson RL, Sedaghat L, Glebova NO, Kurosaki T, Snyder SH. Cytochrome c binds to inositol (1,4,5) trisphosphate receptors, amplifying calcium-dependent apoptosis. Nat Cell Biol. 2003;5:1051–1061. doi: 10.1038/ncb1063. [DOI] [PubMed] [Google Scholar]
- Chan SL, Mattson MP. Caspase and calpain substrates: roles in synaptic plasticity and cell death. J Neurosci Res. 1999;58:167–190. [PubMed] [Google Scholar]
- Chang HY, Yang X. Proteases for cell suicide: functions and regulation of caspases. Microbiol Mol Biol Rev. 2000;64:821–846. doi: 10.1128/mmbr.64.4.821-846.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Nagayama T, Jin K, Stetler RA, Zhu RL, Graham SH, Simon RP. Induction of caspase-3-like protease may mediate delayed neuronal death in the hippocampus after transient cerebral ischemia. J Neurosci. 1998;18:4914–4928. doi: 10.1523/JNEUROSCI.18-13-04914.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng HY, Clayton DF. Activation and habituation of extracellular signal-regulated kinase phosphorylation in zebra finch auditory forebrain during song presentation. J Neurosci. 2004;24:7503–7513. doi: 10.1523/JNEUROSCI.1405-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew SJ, Mello C, Nottebohm F, Jarvis E, Vicario DS. Decrements in auditory responses to a repeated conspecific song are long-lasting and require two periods of protein synthesis in the songbird forebrain. Proc Natl Acad Sci USA. 1995;92:3406–3410. doi: 10.1073/pnas.92.8.3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew SJ, Vicario DS, Nottebohm F. A large-capacity memory system that recognizes calls and songs of individual birds. Proc Natl Acad Sci USA. 1996;93:1950–1955. doi: 10.1073/pnas.93.5.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton DF. The Genomic Action Potential. Neurobiol Learn Mem. 2000;74:185–216. doi: 10.1006/nlme.2000.3967. [DOI] [PubMed] [Google Scholar]
- Clayton DF. Molecular neurobiology of birdsong. In: Blaustein JD, editor. Handbook of Neurochemistry and Molecular Neurobiology. Vol. 21. New York: Kluwer; in press. [Google Scholar]
- Dash PK, Blum S, Moore AN. Caspase activity plays an essential role in long-term memory. Neuroreport. 2000;11:2811–2816. doi: 10.1097/00001756-200008210-00040. [DOI] [PubMed] [Google Scholar]
- Davoli MA, Fourtounis J, Tam J, Xanthoudakis S, Nicholson D, Robertson GS, Ng GY, Xu D. Immunohistochemical and biochemical assessment of caspase-3 activation and DNA fragmentation following transient focal ischemia in the rat. Neuroscience. 2002;115:125–136. doi: 10.1016/s0306-4522(02)00376-7. [DOI] [PubMed] [Google Scholar]
- Dudai Y. The neurobiology of consolidations, or, how stable is the engram? Annual Review of Psychology Annu Rev Psychol. 2004;55:51–86. doi: 10.1146/annurev.psych.55.090902.142050. [DOI] [PubMed] [Google Scholar]
- Faleiro L, Kobayashi R, Fearnhead H, Lazebnik Y. Multiple species of CPP32 and Mch2 are the major active caspases present in apoptotic cells. EMBO J. 1997;16:2271–2281. doi: 10.1093/emboj/16.9.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I, Planas AM. Signaling of cell death and cell survival following focal cerebral ischemia: life and death struggle in the penumbra. J Neuropathol Exp Neurol. 2003;62:329–339. doi: 10.1093/jnen/62.4.329. [DOI] [PubMed] [Google Scholar]
- Frey U, Morris RGM. Synaptic tagging - implications for late maintenance of hippocampal long-term potentiation. Trends Neurosci. 1998;21:181–188. doi: 10.1016/s0166-2236(97)01189-2. [DOI] [PubMed] [Google Scholar]
- Gamblin TC, Chen F, Zambrano A, Abraha A, Lagalwar S, Guillozet AL, Lu M, Fu Y, Garcia-Sierra F, LaPointe N, Miller R, Berry RW, Binder LI, Cryns VL. Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer's disease. Proc Natl Acad Sci U S A. 2003;100:10032–10037. doi: 10.1073/pnas.1630428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemma C, Fister M, Hudson C, Bickford PC. Improvement of memory for context by inhibition of caspase-1 in aged rats. Eur J Neurosci. 2005;22:1751–1756. doi: 10.1111/j.1460-9568.2005.04334.x. [DOI] [PubMed] [Google Scholar]
- Glazner GW, Chan SL, Lu C, Mattson MP. Caspase-mediated degradation of AMPA receptor subunits: a mechanism for preventing excitotoxic necrosis and ensuring apoptosis. J Neurosci. 2000;20:3641–3649. doi: 10.1523/JNEUROSCI.20-10-03641.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyaeva NV, Kudryashov IE, Kudryashova IV. Caspase activity is essential for long-term potentiation. J Neurosci Res. 2003;73:853–864. doi: 10.1002/jnr.10730. [DOI] [PubMed] [Google Scholar]
- Guzowski JF. Insights into immediate-early gene function in hippocampal memory consolidation using antisense oligonucleotide and fluorescent imaging approaches. Hippocampus. 2002;12:86–104. doi: 10.1002/hipo.10010. [DOI] [PubMed] [Google Scholar]
- Huang Y, Rich RL, Myszka DG, Wu H. Requirement of both the second and third BIR domains for the relief of X-linked inhibitor of apoptosis protein (XIAP)-mediated caspase inhibition by Smac. J Biol Chem. 2003;278:49517–49522. doi: 10.1074/jbc.M310061200. [DOI] [PubMed] [Google Scholar]
- Jarskog LF, Gilmore JH, Glantz LA, Gable KL, German TT, Tong RI, Lieberman JA. Caspase-3 Activation in Rat Frontal Cortex Following Treatment with Typical and Atypical Antipsychotics. Neuropsychopharmacology. 2006 doi: 10.1038/sj.npp.1301074. [DOI] [PubMed] [Google Scholar]
- Keane RW, Srinivasan A, Foster LM, Testa MP, Ord T, Nonner D, Wang HG, Reed JC, Bredesen DE, Kayalar C. Activation of CPP32 during apoptosis of neurons and astrocytes. J Neurosci Res. 1997;48:168–180. doi: 10.1002/(sici)1097-4547(19970415)48:2<168::aid-jnr9>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Korhonen L, Napankangas U, Steen H, Chen Y, Martinez R, Lindholm D. Differential regulation of X-chromosome-linked inhibitor of apoptosis protein (XIAP) and caspase-3 by NMDA in developing hippocampal neurons; involvement of the mitochondrial pathway in NMDA-mediated neuronal survival. Exp Cell Res. 2004;295:290–299. doi: 10.1016/j.yexcr.2003.12.026. [DOI] [PubMed] [Google Scholar]
- Kruse AA, Stripling R, Clayton DF. Context-specific habituation of the zenk gene response to song in adult zebra finches. Neurobiol Learn Mem. 2004;82:99–108. doi: 10.1016/j.nlm.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Kruse AA, Stripling R, Clayton DF. Minimal experience required for immediate-early gene induction in zebra finch neostriatum. Neurobiol Learn Mem. 2000;74:179–184. doi: 10.1006/nlme.2000.3968. [DOI] [PubMed] [Google Scholar]
- Kudryashov IE, Yakovlev AA, Kudryashova IV, Gulyaeva NV. Inhibition of caspase-3 blocks long-term potentiation in hippocampal slices. Neurosci Behav Physiol. 2004;34:877–880. doi: 10.1023/b:neab.0000042571.86110.28. [DOI] [PubMed] [Google Scholar]
- Lu C, Fu W, Salvesen GS, Mattson MP. Direct cleavage of AMPA receptor subunit GluR1 and suppression of AMPA currents by caspase-3: implications for synaptic plasticity and excitotoxic neuronal death. Neuromolecular Med. 2002;1:69–79. doi: 10.1385/NMM:1:1:69. [DOI] [PubMed] [Google Scholar]
- Lugli G, Larson J, Martone ME, Jones Y, Smalheiser NR. Dicer and eIF2c are enriched at postsynaptic densities in adult mouse brain and are modified by neuronal activity in a calpain-dependent manner. J Neurochem. 2005;94:896–905. doi: 10.1111/j.1471-4159.2005.03224.x. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Chan SL. Calcium orchestrates apoptosis. Nat Cell Biol. 2003;5:1041–1043. doi: 10.1038/ncb1203-1041. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Duan W. "Apoptotic" biochemical cascades in synaptic compartments: roles in adaptive plasticity and neurodegenerative disorders. J Neurosci Res. 1999;58:152–166. [PubMed] [Google Scholar]
- Mattson MP, Duan W, Pedersen WA, Culmsee C. Neurodegenerative disorders and ischemic brain diseases. Apoptosis. 2001;6:69–81. doi: 10.1023/a:1009676112184. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Keller JN, Begley JG. Evidence for synaptic apoptosis. Exp Neurol. 1998;153:35–48. doi: 10.1006/exnr.1998.6863. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory — a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- Mello CV. Mapping vocal communication pathways in birds with inducible gene expression. Journal of Comparative Physiology A, Neuroethology, Sensory, Neural, and Behavioral Physiology. 2002;188:943–959. doi: 10.1007/s00359-002-0347-1. [DOI] [PubMed] [Google Scholar]
- Mello CV, Ribeiro S. Zenk protein regulation by song in the brain of songbirds. J Comp Neurol. 1998;393:426–438. doi: 10.1002/(sici)1096-9861(19980420)393:4<426::aid-cne3>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Mello CV, Clayton DF. Song-induced ZENK gene expression in auditory pathways of songbird brain and its relation to the song control system. J Neurosci. 1994;14:6652–6666. doi: 10.1523/JNEUROSCI.14-11-06652.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello CV, Nottebohm F, Clayton DF. Repeated exposure to one song leads to a rapid and persistent decline in an immediate early gene's response to that song in zebra finch telencephalon. J Neurosci. 1995;15:6919–6925. doi: 10.1523/JNEUROSCI.15-10-06919.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello CV, Vicario DS, Clayton DF. Song presentation induces gene expression in the songbird forebrain. Proc Natl Acad Sci USA. 1992;89:6818–6822. doi: 10.1073/pnas.89.15.6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millin PM, Moody EW, Riccio DC. Interpretations of retrograde amnesia: old problems redux. Nature Reviews Neuroscience. 2001;2:68–70. doi: 10.1038/35049075. [DOI] [PubMed] [Google Scholar]
- Namura S, Zhu J, Fink K, Endres M, Srinivasan A, Tomaselli KJ, Yuan J, Moskowitz MA. Activation and cleavage of caspase-3 in apoptosis induced by experimental cerebral ischemia. J Neurosci. 1998;18:3659–3668. doi: 10.1523/JNEUROSCI.18-10-03659.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan ML, Pytte CL, Vicario DS. Early auditory experience generates long-lasting memories that may subserve vocal learning in songbirds. Proc Natl Acad Sci USA. 2006;103:1088–1093. doi: 10.1073/pnas.0510136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson GS, Crocker SJ, Nicholson DW, Schulz JB. Neuroprotection by the inhibition of apoptosis. Brain Pathol. 2000;10:283–292. doi: 10.1111/j.1750-3639.2000.tb00262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito A, Hayashi T, Okuno S, Ferrand-Drake M, Chan PH. Interaction between XIAP and Smac/DIABLO in the mouse brain after transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2003;23:1010–1019. doi: 10.1097/01.WCB.0000080702.47016.FF. [DOI] [PubMed] [Google Scholar]
- Slagsvold HH, Rosseland CM, Jacobs C, Khuong E, Kristoffersen N, Gaarder M, Fallgren AB, Huitfeldt HS, Paulsen RE. High molecular weight DNA fragments are processed by caspase sensitive or caspase independent pathways in cultures of cerebellar granule neurons. Brain Res. 2003;984:111–121. doi: 10.1016/s0006-8993(03)03119-6. [DOI] [PubMed] [Google Scholar]
- Springer JE, Azbill RD, Knapp PE. Activation of the caspase-3 apoptotic cascade in traumatic spinal cord injury. Nat Med. 1999;5:943–946. doi: 10.1038/11387. [DOI] [PubMed] [Google Scholar]
- Stepanichev MY, Kudryashova IV, Yakovlev AA, Onufriev MV, Khaspekov LG, Lyzhin AA, Lazareva NA, Gulyaeva NV. Central administration of a caspase inhibitor impairs shuttle-box performance in rats. Neuroscience. 2005;136:579–591. doi: 10.1016/j.neuroscience.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Stoddard PK. In: Vocal recognition of neighbors by territorial passerines. Kroodsma DE, Miller EH, editors. Ithaca, NY: Cornell University Press; 1996. pp. 356–374. [Google Scholar]
- Stripling R, Kruse AA, Clayton DF. Development of song responses in the zebra finch caudomedial neostriatum: Role of genomic and electrophysiological activities. J Neurobiol. 2001;48:163–180. doi: 10.1002/neu.1049. [DOI] [PubMed] [Google Scholar]
- Stripling R, Kruse AA, Clayton DF. Habituation of molecular and behavioral responses to song in adult male and female zebra finches. submitted. [Google Scholar]
- Stripling R, Milewski L, Kruse AA, Clayton DF. Rapidly learned song-discrimination without behavioral reinforcement in adult male zebra finches (Taeniopygia guttata) Neurobiol Learn Mem. 2003;79:41–50. doi: 10.1016/s1074-7427(02)00005-9. [DOI] [PubMed] [Google Scholar]
- Stripling R, Volman S, Clayton D. Response modulation in the zebra finch caudal neostriatum: Relationship to nuclear gene regulation. J Neurosci. 1997;17:3883–3893. doi: 10.1523/JNEUROSCI.17-10-03883.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi R, Deveraux Q, Tamm I, Welsh K, Assa-Munt N, Salvesen GS, Reed JC. A single BIR domain of XIAP sufficient for inhibiting caspases. J Biol Chem. 1998;273:7787–7790. doi: 10.1074/jbc.273.14.7787. [DOI] [PubMed] [Google Scholar]
- Tchernichovski O, Mitra PP, Lints T, Nottebohm F. Dynamics of the vocal imitation process: how a zebra finch learns its song. Science. 2001;291:2564–2569. doi: 10.1126/science.1058522. [DOI] [PubMed] [Google Scholar]
- Wain HM, Lush M, Ducluzeau F, Povey S. Genew: the human gene nomenclature database. Nucleic Acids Res. 2002;30:169–171. doi: 10.1093/nar/30.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wain HM, Lush MJ, Ducluzeau F, Khodiyar VK, Povey S. Genew: the Human Gene Nomenclature Database, 2004 updates. Nucleic Acids Res. 2004;32:D255–7. doi: 10.1093/nar/gkh072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KK. Calpain and caspase: can you tell the difference? Trends Neurosci. 2000;23:20–26. doi: 10.1016/s0166-2236(99)01536-2. [DOI] [PubMed] [Google Scholar]
- Zhuang S, Lynch MC, Kochevar IE. Caspase-8 mediates caspase-3 activation and cytochrome c release during singlet oxygen-induced apoptosis of HL-60 cells. Exp Cell Res. 1999;250:203–212. doi: 10.1006/excr.1999.4501. [DOI] [PubMed] [Google Scholar]


