Abstract
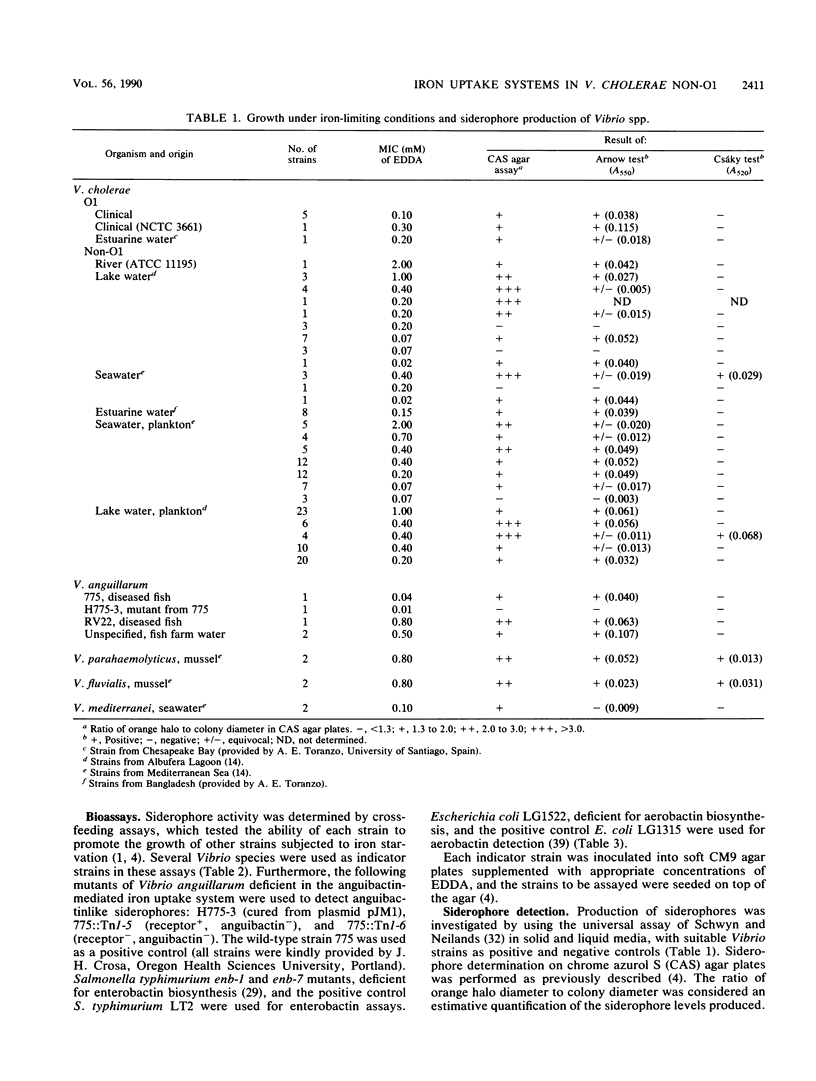
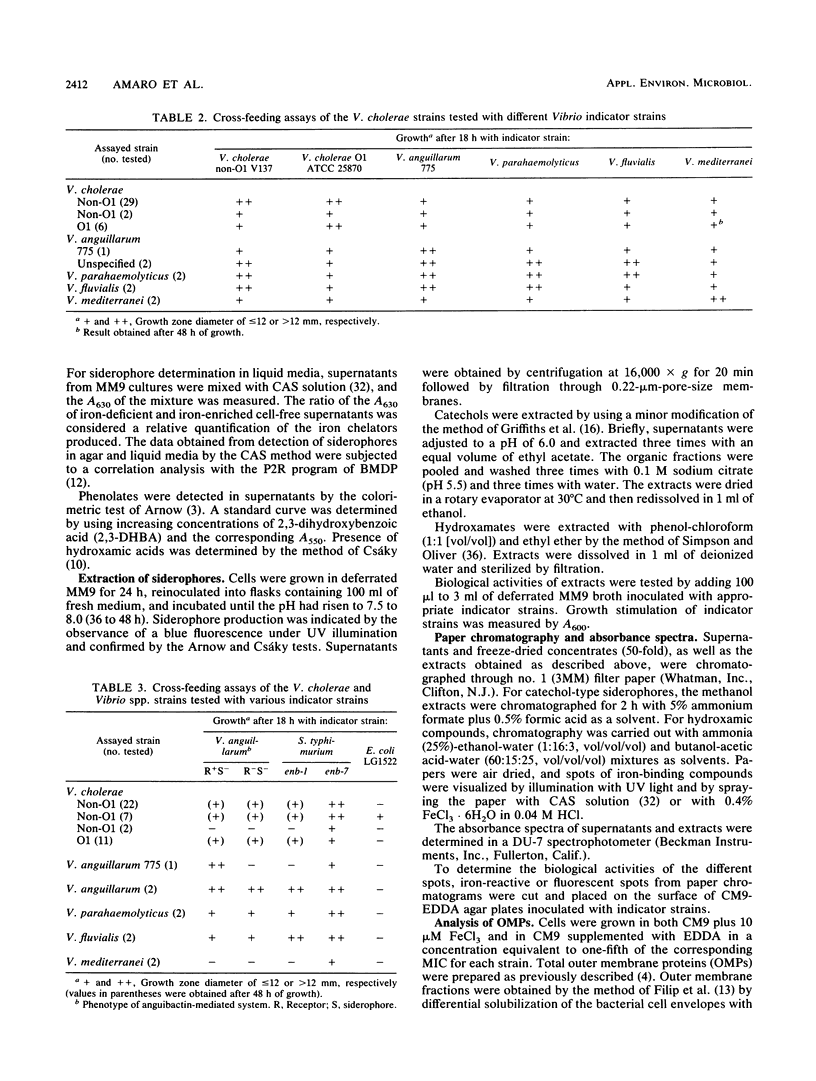
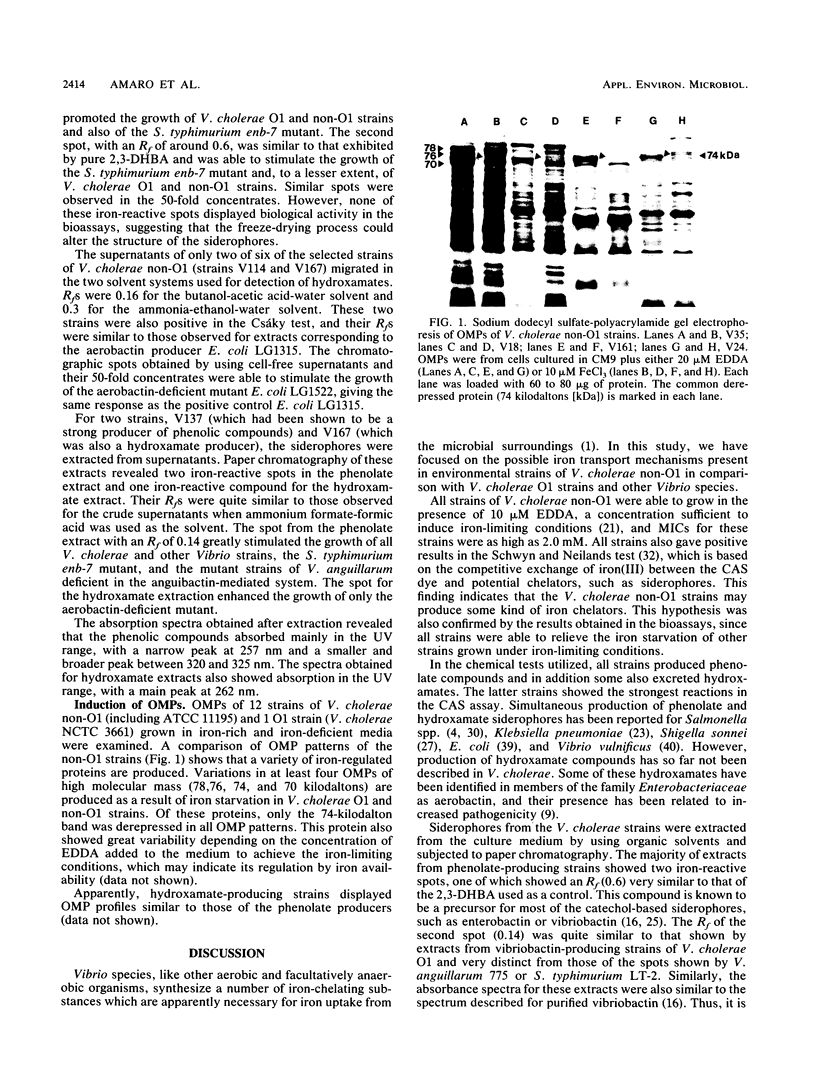
A total of 156 strains of Vibrio cholerae non-O1 from aquatic origins were examined for the presence of iron uptake mechanisms and compared with O1 strains and other Vibrio species. All non-O1 strains were able to grow in iron-limiting conditions, with MICs of ethylenediaminedi (O-hydroxyphenylacetic acid) ranging from 20 microM to 2 mM. The production of siderophores was demonstrated by growth in chrome azurol S agar and cross-feeding assays. All strains produced phenolate-type compounds, as assessed by the chemical tests and by bioassays with Salmonella typhimurium enb-7. Some of the strains also promoted the growth of S. typhimurium enb-1 (which can use only enterobactin as a siderophore) as well as some strains of Vibrio anguillarum deficient in the anguibactin-mediated system. The chromatographic analyses and absorption spectra of siderophores extracted from culture supernatants suggest that vibriobactin may be produced by the strains examined. Interestingly, some strains also produced hydroxamate-type compounds, as determined by chemical tests, and were able to promote the growth of an aerobactin-deficient strain of Escherichia coli. These results were confirmed by the absorption spectra and chromatographic analyses of the culture extracts. The synthesis of iron-regulated outer membrane proteins in representative strains was also examined. The molecular sizes of the main induced proteins ranged from 70 to 78 kilodaltons. These results indicate that several iron uptake mechanisms which could be involved in environmental survival and pathogenicity are present in environmental V. cholerae non-O1 strains.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aznar R., Amaro C., Alcaide E., Lemos M. L. Siderophore production by environmental strains of Salmonella species. FEMS Microbiol Lett. 1989 Jan 1;48(1):7–12. doi: 10.1016/0378-1097(89)90137-7. [DOI] [PubMed] [Google Scholar]
- Blake P. A., Allegra D. T., Snyder J. D., Barrett T. J., McFarland L., Caraway C. T., Feeley J. C., Craig J. P., Lee J. V., Puhr N. D. Cholera--a possible endemic focus in the United States. N Engl J Med. 1980 Feb 7;302(6):305–309. doi: 10.1056/NEJM198002073020601. [DOI] [PubMed] [Google Scholar]
- Blake P. A., Weaver R. E., Hollis D. G. Diseases of humans (other than cholera) caused by vibrios. Annu Rev Microbiol. 1980;34:341–367. doi: 10.1146/annurev.mi.34.100180.002013. [DOI] [PubMed] [Google Scholar]
- Booth B. A., Finkelstein R. A. Presence of hemagglutinin/protease and other potential virulence factors in O1 and non-O1 Vibrio cholerae. J Infect Dis. 1986 Jul;154(1):183–186. doi: 10.1093/infdis/154.1.183. [DOI] [PubMed] [Google Scholar]
- Bullen J. J. The significance of iron in infection. Rev Infect Dis. 1981 Nov-Dec;3(6):1127–1138. doi: 10.1093/clinids/3.6.1127. [DOI] [PubMed] [Google Scholar]
- Carbonetti N. H., Boonchai S., Parry S. H., Väisänen-Rhen V., Korhonen T. K., Williams P. H. Aerobactin-mediated iron uptake by Escherichia coli isolates from human extraintestinal infections. Infect Immun. 1986 Mar;51(3):966–968. doi: 10.1128/iai.51.3.966-968.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta-Roy K., Banerjee K., De S. P., Ghose A. C. Comparative study of expression of hemagglutinins, hemolysins, and enterotoxins by clinical and environmental isolates of non-O1 Vibrio cholerae in relation to their enteropathogenicity. Appl Environ Microbiol. 1986 Oct;52(4):875–879. doi: 10.1128/aem.52.4.875-879.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garay E., Arnau A., Amaro C. Incidence of Vibrio cholerae and related vibrios in a coastal lagoon and seawater influenced by lake discharges along an annual cycle. Appl Environ Microbiol. 1985 Aug;50(2):426–430. doi: 10.1128/aem.50.2.426-430.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G. L., Sigel S. P., Payne S. M., Neilands J. B. Vibriobactin, a siderophore from Vibrio cholerae. J Biol Chem. 1984 Jan 10;259(1):383–385. [PubMed] [Google Scholar]
- Kaper J., Lockman H., Colwell R. R., Joseph S. W. Ecology, serology, and enterotoxin production of Vibrio cholerae in Chesapeake Bay. Appl Environ Microbiol. 1979 Jan;37(1):91–103. doi: 10.1128/aem.37.1.91-103.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaysner C. A., Abeyta C., Jr, Wekell M. M., DePaola A., Jr, Stott R. F., Leitch J. M. Incidence of Vibrio cholerae from estuaries of the United States West Coast. Appl Environ Microbiol. 1987 Jun;53(6):1344–1348. doi: 10.1128/aem.53.6.1344-1348.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lemos M. L., Salinas P., Toranzo A. E., Barja J. L., Crosa J. H. Chromosome-mediated iron uptake system in pathogenic strains of Vibrio anguillarum. J Bacteriol. 1988 Apr;170(4):1920–1925. doi: 10.1128/jb.170.4.1920-1925.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles A. A., Khimji P. L. Enterobacterial chelators of iron: their occurrence, detection, and relation to pathogenicity. J Med Microbiol. 1975 Nov;8(4):477–490. doi: 10.1099/00222615-8-4-477. [DOI] [PubMed] [Google Scholar]
- Miller J. F., Mekalanos J. J., Falkow S. Coordinate regulation and sensory transduction in the control of bacterial virulence. Science. 1989 Feb 17;243(4893):916–922. doi: 10.1126/science.2537530. [DOI] [PubMed] [Google Scholar]
- Nassif X., Sansonetti P. J. Correlation of the virulence of Klebsiella pneumoniae K1 and K2 with the presence of a plasmid encoding aerobactin. Infect Immun. 1986 Dec;54(3):603–608. doi: 10.1128/iai.54.3.603-608.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilands J. B. Microbial iron compounds. Annu Rev Biochem. 1981;50:715–731. doi: 10.1146/annurev.bi.50.070181.003435. [DOI] [PubMed] [Google Scholar]
- O'Brien I. G., Cox G. B., Gibson F. Biologically active compounds containing 2,3-dihydroxybenzoic acid and serine formed by Escherichia coli. Biochim Biophys Acta. 1970 Mar 24;201(3):453–460. doi: 10.1016/0304-4165(70)90165-0. [DOI] [PubMed] [Google Scholar]
- Payne S. M., Finkelstein R. A. Siderophore production by Vibrio cholerae. Infect Immun. 1978 Apr;20(1):310–311. doi: 10.1128/iai.20.1.310-311.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikaytis B. D., Carlone G. M., Edmonds P., Mayer L. W. Robust estimation of standard curves for protein molecular weight and linear-duplex DNA base-pair number after gel electrophoresis. Anal Biochem. 1986 Feb 1;152(2):346–364. doi: 10.1016/0003-2697(86)90420-3. [DOI] [PubMed] [Google Scholar]
- Pollack J. R., Ames B. N., Neilands J. B. Iron transport in Salmonella typhimurium: mutants blocked in the biosynthesis of enterobactin. J Bacteriol. 1970 Nov;104(2):635–639. doi: 10.1128/jb.104.2.635-639.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabsch W., Reissbrodt R. Investigations of Salmonella strains from different clinical-epidemiological origin with phenolate and hydroxamate (aerobactin)--siderophore bioassays. J Hyg Epidemiol Microbiol Immunol. 1988;32(3):353–360. [PubMed] [Google Scholar]
- Schwyn B., Neilands J. B. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987 Jan;160(1):47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- Sciortino C. V., Finkelstein R. A. Vibrio cholerae expresses iron-regulated outer membrane proteins in vivo. Infect Immun. 1983 Dec;42(3):990–996. doi: 10.1128/iai.42.3.990-996.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigel S. P., Payne S. M. Effect of iron limitation on growth, siderophore production, and expression of outer membrane proteins of Vibrio cholerae. J Bacteriol. 1982 Apr;150(1):148–155. doi: 10.1128/jb.150.1.148-155.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigel S. P., Stoebner J. A., Payne S. M. Iron-vibriobactin transport system is not required for virulence of Vibrio cholerae. Infect Immun. 1985 Feb;47(2):360–362. doi: 10.1128/iai.47.2.360-362.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson L. M., Oliver J. D. Siderophore production by Vibrio vulnificus. Infect Immun. 1983 Aug;41(2):644–649. doi: 10.1128/iai.41.2.644-649.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoebner J. A., Payne S. M. Iron-regulated hemolysin production and utilization of heme and hemoglobin by Vibrio cholerae. Infect Immun. 1988 Nov;56(11):2891–2895. doi: 10.1128/iai.56.11.2891-2895.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Tiel-Menkveld G. J., Mentjox-Vervuurt J. M., Oudega B., de Graaf F. K. Siderophore production by Enterobacter cloacae and a common receptor protein for the uptake of aerobactin and cloacin DF13. J Bacteriol. 1982 May;150(2):490–497. doi: 10.1128/jb.150.2.490-497.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. H. Novel iron uptake system specified by ColV plasmids: an important component in the virulence of invasive strains of Escherichia coli. Infect Immun. 1979 Dec;26(3):925–932. doi: 10.1128/iai.26.3.925-932.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]



