Abstract
At a time when the twin epidemics of obesity and type 2 diabetes threaten to engulf even the most well-resourced Western healthcare systems, the nuclear receptor peroxisome proliferator-activated receptor γ (PPARγ) has emerged as a bona fide therapeutic target for treating human metabolic disease. The novel insulin-sensitizing antidiabetic thiazolidinediones (TZDs, e.g., rosiglitazone, pioglitazone), which are licensed for use in the treatment of type 2 diabetes, are high-affinity PPARγ ligands, whose beneficial effects extend beyond improvement in glycaemic control to include amelioration of dyslipidaemia, lowering of blood pressure, and favourable modulation of macrophage lipid handling and inflammatory responses. However, a major drawback to the clinical use of exisiting TZDs is weight gain, reflecting both enhanced adipogenesis and fluid retention, neither of which is desirable in a population that is already overweight and prone to cardiovascular disease. Accordingly, the “search is on” to identify the next generation of PPARγ modulators that will promote maximal clinical benefit by targeting specific facets of the metabolic syndrome (glucose intolerance/diabetes, dyslipidaemia, and hypertension), while simultaneously avoiding undesirable side effects of PPARγ activation (e.g., weight gain). This paper outlines the important clinical and laboratory observations made in human subjects harboring genetic variations in PPARγ that support such a therapeutic strategy.
1. INTRODUCTION
The health of a nation has long been recognized to be a function of its wealth. Traditionally, countries with limited resources have struggled to eradicate diseases that are often considered a thing of the past in so-called “developed” or “industrialized” nations. However, in recent years it has become clear that wealth does not always equate with good health. Indeed, we now face the very real possibility that in the first half of this century, average life expectancy in industrialized countries such as the US and UK will plateau or decline, despite continuing economic growth and prosperity [1]. The obesity epidemic, which is currently sweeping through “Western civilization,” is undoubtedly the single biggest factor behind this “unwanted reversal” [1]. Recent figures from the US reveal an alarming 75% increase in the prevalence of obesity over the past 25 years, such that a third of the population is now officially obese, that is to say, at least 20% heavier than their ideal weight [2]. Many Western European countries and Japan are not far behind. Obesity is a major risk factor for insulin resistance, type 2 diabetes mellitus (T2DM), hypertension, and dyslipidaemia (particularly hypertriglyceridaemia and low high-density lipoprotein cholesterol (HDL-C)); this cluster of medical sequelae is often grouped together under the umbrella term “metabolic syndrome,” and over the past decade the thresholds that must be met for the diagnosis of this entity have been progressively refined, culminating most recently in a consensus statement from the International Diabetes Federation (Table 1). Not surprisingly, subjects who meet the diagnostic criteria for this disorder are at significantly increased risk of atherosclerotic cardiovascular disease (reviewed in [3]).
Table 1.
Diagnostic criteria for the human metabolic syndrome. WHO, World Health Organization; EGIR, European Group for the Study of Insulin Resistance; NCEP ATP III, National Cholesterol Education Program Adult Treatment Panel III; IDF, International Diabetes Federation; T2DM, type 2 diabetes mellitus; IGT, impaired glucose tolerance; IR, insulin resistance; TG, triglycerides; HDL, high density lipoprotein cholesterol; BP, blood pressure; BMI, body mass index; WHR, waist hip ratio; WC, waist circumference; AER, albumin excretion rate; M, male; F, female.
| WHO, 1999 | EGIR, 1999 | NCEP ATP III, 2001 | IDF, 2005 |
|
| |||
| T2DM or IGT or IR | IR or hyperinsulinaemia, in nondiabetic subjects | Central obesity: WC ≥ ethnicity specific cut-offs | |
| with ≥2 of the following | with ≥2 of the following | ≥3 of the following | with ≥2 of the following |
|
| |||
| Hyperglycaemia | Hyperglycaemia | Hyperglycaemia | |
| Fasting plasma glucose ≥ | Fasting plasma glucose ≥ | Fasting plasma glucose ≥ | |
| 6.1 mmol/L, but nondiabetic | 6.1 mmol/L or | 5.6 mmol/L or | |
| treated with antidiabetic medication. | previously diagnosed T2DM | ||
|
| |||
| Dyslipidaemia | Dyslipidaemia | Hypertriglyceridaemia | Hypertriglyceridaemia |
| TG >1.7 mmol/L and/or | TG >2.0 mmol/L or | TG ≥1.7 mmol/L | TG >1.7 mmol/L or |
| HDL <0.9 mmol/L (M) | HDL <1.0 mmol/L or | treated for this lipid abnormality | |
| HDL <1.0 mmol/L (F) | treated for dyslipidaemia | ||
|
| |||
| Low HDL cholesterol | Reduced HDL cholesterol | ||
| HDL <1.0 mmol/L (M) | HDL <1.03 mmol/L (M) | ||
| HDL <1.3 mmol/L (F) | HDL <1.29 mmol/L (F) or | ||
| treated for this lipid abnormality | |||
|
| |||
| Hypertension | Hypertension | Hypertension | Hypertension |
| BP ≥140/90 mmHg±medication | BP ≥140/90 mmHg or | BP ≥130/85 mmHg or | BP ≥130/85 mmHg or |
| treated for hypertension | treated for hypertension | treated for hypertension | |
|
| |||
| Obesity | Central obesity | Central obesity | Central obesity |
| BMI ≥30 kg/m2 or | WC ≥94 cm (M) | WC ≥102 cm (M) | See above—core requirement for |
| WHR >0.9 (M) | WC ≥80 cm (F) | WC ≥88 cm (F) | diagnosis of syndrome |
| WHR >0.85 (F) | |||
|
| |||
| Microalbuminuria | |||
| Urinary AER >20 mcg/min | |||
So how can we arrest/reverse this apparently relentless march towards “metabolic meltdown”? The solution seems obvious: more effective obesity prevention and treatment. Limiting caloric intake and increasing energy expenditure to promote neutral (or in obese subjects negative) rather than positive energy balance is likely to yield enormous benefits at both the individual and population levels. Indeed, “lifestyle intervention” studies have already convincingly demonstrated that the risk of developing complications such as T2DM can be significantly reduced using such an approach [4, 5]. Unfortunately however, while this is a laudable goal, most clinicians know only too well that in practice it is very difficult to achieve/sustain, and hence attention has turned towards seeking novel therapies that are capable of ameliorating/reversing weight gain, insulin resistance, and their unwanted sequelae. Understanding the genes that are involved in maintaining metabolic homeostasis in the face of differing nutritional and environmental stresses is essential to the rational development of these strategies.
In recent years, a group of transcription factors belonging to the nuclear receptor superfamily has emerged as key players in the regulation of mammalian metabolism. Peroxisome proliferator-activated receptor γ(PPARγ) is perhaps the best characterized of these so-called metabolic nuclear receptors, serving as it does to integrate the control of energy, glucose, and lipid homeostasis. The activity of PPARγ is governed by the binding of small lipophilic ligands, principally fatty acids, derived from nutrition or metabolism [6, 7], and activation of the receptor is a critical step in the pathway to adipocyte differentiation and fat cell maturation. Hence, it is easy to envisage how chronic exposure to high levels of dietary PPARγ ligands (provided in abundance in the Western diet) could promote the development of obesity, insulin resistance, and metabolic dysfunction, and why receptor modulation might offer a route to prevention/amelioration of these important cardiovascular risk factors. Indeed, drugs targeting PPARγ activity (thiazolidinediones (TZDs), e.g., rosiglitazone, pioglitazone) are already in widespread clinical use as effective antidiabetic agents, enhancing insulin sensitivity, elevating high-density lipoprotein cholesterol (HDL-C) levels, and lowering blood pressure [8]. Importantly other studies have begun to examine whether these agents actually lower cardiovascular event rates [9], and if they are capable of reducing the risk of progression to overt T2DM in those with existing impaired glucose regulation [10].
Paradoxically however, TZDs actually promote weight gain rather than weight loss. A significant part of this increase can be attributed to enhanced adipogenesis, consistent with TZDs acting as high-affinity agonists for PPARγ [11–13]. In addition, fluid retention and expansion of the extracellular compartment (possibly through altered renal sodium handling [14]) may contribute to weight gain in some patients, especially those with preexisting cardiac impairment [15]. Together, these observations raise an important question: is it possible to develop more selective PPARγ modulators, with even greater potential to improve metabolic dysfunction, yet at the same time with reduced propensity to cause weight gain and fluid retention? Clearly, the answer to this question is dependent on the basic biology of PPARγ and whether it proves possible to regulate receptor function in a tissue- and a target-gene-specific manner.
This paper summarizes the important contributions that human genetic studies have made to our understanding of the role of PPARγ in the regulation of mammalian metabolic homeostasis, emphasizing the potential benefits and limitations that we can expect from more targeted approaches to modulating receptor function, and thus ensuring that in an era marked by an increasing prevalence of obesity, diabetes and cardiovascular disease, PPARγ remains more of “a help” than “a hindrance.”
2. PPARγ-STRUCTURE, FUNCTION, AND LIGAND REGULATION
The human nuclear receptor superfamily comprises 48 ligand-inducible transcription factors that respond to a variety of stimuli including steroid and thyroid hormones, vitamins, lipid metabolites, and xenobiotics. PPARγ is the third member of a subdivision within the superfamily that also includes PPARα and PPARδ [25, 26]. Together, the PPARs function as key transcriptional regulators that govern metabolic homeostasis by serving as lipid sensors, responding to dietary fatty acids and their derivatives. However, each has a distinct pattern of tissue expression, and consistent with this, specific roles in the regulation of energy metabolism (reviewed in [25, 26]). The importance of these receptors in physiology and disease is evidenced by the fact that PPARα and PPARγ are the molecular targets for the lipid-lowering fibrate class of drugs and TZDs, respectively, while PPARδ ligands are currently being developed in anticipation that they will offer a novel approach to tackling obesity and metabolic dysfunction through effects on energy expenditure, HDL-C metabolism, and macrophage inflammatory responses (reviewed in [26]).
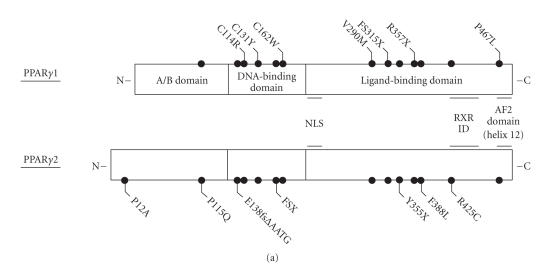
Differential promoter usage, coupled with alternate splicing of the PPARG gene, generates two protein isoforms: PPARγ2, expressed from a single γ2 promoter, contains an additional 28 N-terminal amino acids and is nearly adipose-specific; PPARγ1, whose expression can be regulated by multiple (γ1, γ3, γ4) promoters, is more ubiquitously distributed [27–29]. Like other nuclear receptors, PPARγ exhibits a modular structure consisting of distinct functional domains: the N-terminal A/B domain harbors a ligand-independent transcriptional activation function (AF1), which is stronger for the γ2 than γ1 isoform; the central DNA-binding domain, containing two zinc finger motifs, facilitates interaction with specific binding sites (PPAR response elements (PPREs)) in target gene promoters; the larger C-terminal domain mediates ligand-binding, heterodimerization with the retinoid X receptor (RXR), and contains a powerful ligand-dependent activation (AF2) function (Figure 1(a)).
Figure 1.
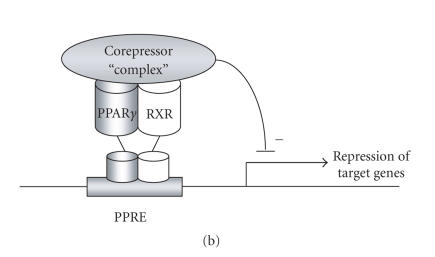
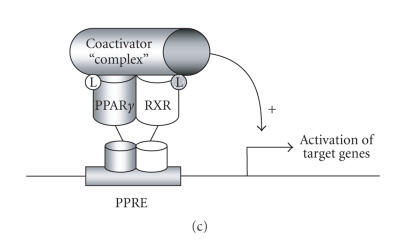
Structure function of PPARγ. (a) Schematic representation of the three principal domains of PPARγ, denoting the positions of several of the natural genetic variants that have been identified in the human receptor. Note that mutations and polymorphisms have been depicted based on the nomenclature (γ1 or γ2) used in the primary publication [16–23]. FSX denotes the mutation (A553ΔAAAiT)fs185(stop186); FS315X denotes the mutation (A935ΔC)fs312(stop315). (b) In the absence of exogenous ligand, PPARγ recruits a corepressor complex to a subset of target genes (e.g., adipocyte glycerol kinase), thereby repressing basal transcription [24]. (c) Addition of ligand induces a conformational change in the receptor, which promotes corepressor release and coactivator recruitment. For other target genes (e.g., aP2), the receptor appears to be constitutively active even in the absence of exogenous ligand [24]. NLS denotes nuclear localization signal; RXR denotes retinoid X receptor; ID denotes interaction domain; AF2 denotes activation function 2; PPRE denotes PPAR response element.
Initially, PPARγ was considered to be a constitutively active receptor, recruiting transcriptional coactivators (e.g., steroid receptor coactivator-1 (SRC-1)) to classical target genes (e.g., adipocyte protein 2 (aP2)) even in the absence of ligand. More recently however, Guan et al. have shown that the unliganded PPARγ/RXR heterodimer can actively silence a subset of genes (e.g., adipocyte glycerol kinase (GyK)), in a manner analogous to that seen with the thyroid hormone (TR) and retinoic acid (RAR) receptors [24] (Figure 1(b)). Transcriptional silencing is mediated through recruitment of a multiprotein corepressor complex, containing either NCoR (nuclear receptor corepressor) or SMRT (silencing mediator of retinoic acid and thyroid receptors), together with histone-modifying enzymes (e.g., histone deacetylase 3 (HDAC 3)), which condense chromatin structure, thus impeding gene transcription. In contrast, binding of cognate or exogenous ligand(s) induces a conformational change in the heterodimer such that it now dissociates from any bound corepressor proteins and instead recruits a coactivator complex, containing histone acetyltransferases (e.g., CREB-binding protein (CBP)), which relaxes the chromatin structure so as to permit greater levels of gene transcription (Figure 1(c)).
A variety of putative endogenous activators has been described for PPARγ, including fatty acids, eicosanoids, and derivatives of oxidized low-density lipoproteins [30]. The prostaglandin J2 derivative 15-deoxy-Δ12,14-PGJ2 is also capable of activating PPARγ in vitro, although it is doubtful as to whether it exists at sufficient concentrations in vivo to serve as a physiological ligand. Recently, Tzameli et al. have reported the existence of an as yet undefined ligand(s) that is produced transiently during adipocyte differentiation [31].
3. PPARγ-A KEY THERAPEUTIC TARGET IN THE HUMAN METABOLIC SYNDROME
Patients with the metabolic syndrome typically require a “cocktail of drugs” to treat the individual components of the disorder and its associated atherosclerotic complications (e.g., oral hypoglycaemic agents, insulin, statins, fibrates, antihypertensives, aspirin, etc.). Unfortunately, many of these drugs confer little benefit in terms of correcting the underlying metabolic disturbance, and indeed some even exacerbate the situation, for example, insulin-induced weight gain. Not surprisingly then, compliance with these complex treatment regimens is often poor.
In contrast, drugs that target PPARγ appear, at least in theory, to offer an attractive and perhaps more logical approach to treating the metabolic syndrome, by virtue of their ability to ameliorate insulin resistance and other facets of the condition [8]. Set against this however is the well-documented increase in body weight that is observed with currently available TZDs [8]. It is these observations that have led scientists and clinicians alike to ask whether it is possible to retain/enhance the metabolic benefits of PPARγ activation, yet at the same time minimize undesirable side effects. The following sections outline the human genetic evidence that supports such a strategy, with specific reference to each of the key components of the metabolic syndrome.
3.1. PPARγ and adipogenesis
in vitro studies suggest that PPARγ is the ultimate effector of adipogenesis in a transcriptional cascade that also involves members of the C/EBP transcription factor family [32]. Modulation of PPARγ expression and/or action in rodent cell lines has conclusively shown that the receptor is both essential and, in the presence of PPARγ agonists, is sufficient for adipogenesis [33]. Consonant with this, PPARγ knockout mice fail to develop adipose tissue [34–36], while their heterozygous counterparts have reduced fat depots [36]. Studies in human tissues point to a similar critical role for PPARγ in the regulation of adipogenesis. Exposure of cultured primary human preadipocytes to PPARγ activators (e.g., TZDs) induces their differentiation [32], while both chemical and biological receptor antagonists efficiently block this process [37].
It comes as no surprise then to learn that human subjects treated with synthetic PPARγ agonists (e.g., rosiglitazone, pioglitazone) gain weight through enhanced adipogenesis [8]. Despite this, metabolic function in the majority of TZD recipients improves. This apparent TZD paradox undoubtedly reflects the ability of these agents to modify adipocyte function and free fatty acid storage in a favorable manner that promotes insulin sensitization; however, it may also be dependent, at least in part, on PPARγ activation mediating depot-specific rather than global changes in adipogenesis. For example, it is notable that the increase in fat mass observed in type 2 diabetics treated with TZDs is not uniformly distributed, with a tendency to accumulate subcutaneous (e.g., limb/gluteal) fat, whereas visceral adipose tissue volume is reduced or unchanged (reviewed in detail in [38]). Consistent with this, preadipocytes isolated from subcutaneous abdominal adipose tissue have been shown in some (although not all) studies to differentiate more readily in response to TZDs than cells from visceral depots taken from the same subjects [39].
3.1.1. Gain- and loss-of-function mutations
With PPARγ agonists promoting adipogenesis, it would seem reasonable to speculate that gain-of-function PPARγ mutations should increase body fat mass. Ristow et al. have provided support for this hypothesis, with the identification of four morbidly obese (BMI 37.9 to 47.2 kg/m2) German subjects, all of whom harbored a gain-of-function mutation (Pro115Gln PPARγ2) within the N-terminal domain of the receptor [40]. The transcriptional activity of PPARγ is subject to modification through phosphorylation of a serine residue at codon 114 [41, 42], and mutation of the adjacent proline was shown to interfere with this process, resulting in a receptor with constitutive transcriptional activity and enhanced adipogenic potential [40]. Subsequently however, a fifth subject, with only a mildly elevated BMI (28.5 kg/m2), was found to carry the same amino acid substitution, which is in marked contrast to the findings of the original study [43]. Thus, for now the significance of this particular genetic variant remains unclear, and further mutation carriers must be identified to confirm whether Pro115Gln does indeed predispose to obesity and, if so, whether there is a depot-specific pattern to the accretion of adipose tissue.
In contrast, there is now a compelling body of data from the study of human subjects with loss-of-function mutations in PPARγ to confirm a pivotal role for this receptor in human adipogenesis. To date, twelve different heterozygous mutations (missense, nonsense, and frameshift) have been identified within the DNA- (DBD) and ligand-binding (LBD) domains of the receptor (Figure 1(a)) [16–23], with functional studies, where available, confirming that the mutant receptors are transcriptionally impaired. In keeping with their dominant mode of inheritance, several of the mutants have also been shown to be capable of inhibiting the activity of their wild-type counterpart in a dominant negative manner, reflecting either aberrant corepressor recruitment to DNA-bound mutant receptors [16, 44], or transcriptional interference through coactivator sequestration by DNA-binding deficient mutants [23]. In contrast, other mutants appear to lack dominant negative activity, with the clinical phenotype purported to be a consequence of haploinsufficiency [18, 20–22]. In keeping with the latter, Al-Shali et al. have recently identified a kindred harboring a novel heterozygous A > G mutation at position −14 within intron B of PPARG (upstream of exon 1), which reduces promoter activity of the PPARγ4 isoform [45]. This mutation cosegregated with a phenotype of partial lipodystrophy and metabolic dysfunction similar to that observed in subjects harboring loss-of-function mutations within the DBD or LBD [45].
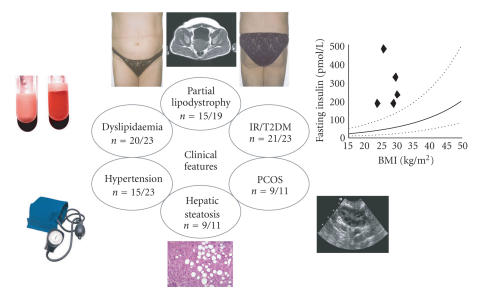
Together, these reports describe more than twenty adult subjects, the majority of whom exhibit a stereotyped pattern of partial lipodystrophy, in which subcutaneous fat is diminished in the limbs and gluteal region, while being preserved/increased in the subcutaneous and visceral abdominal depots (Figure 2) [16–23]. Some phenotypic differences have been observed with facial and neck adipose tissues, which were reported to be increased in individuals from two kindreds, but normal or reduced in most other cases [16–23]. These findings are again strongly suggestive of a depot-specific role for PPARγ in human adipogenesis, and complement the observations made in diabetic subjects receiving TZD treatment. Clearly one challenge is to understand why visceral adipose tissue appears relatively refractory to PPARγ regulation despite expressing comparable levels of receptor to its subcutaneous counterpart. Studies of fat biopsies from different depots in PPARγ mutation carriers might offer a unique route to addressing this important question.
Figure 2.
Clinical features exhibited by adult subjects harboring loss-of-function mutations in human PPARγ. For each parameter shown, the numerator denotes the reported number of affected individuals, and the denominator denotes the number of subjects for whom relevant information is available.
Interestingly, a transgenic knockin mouse model based on the human Pro467Leu mutation (Pro465Leu) has recently been reported by two independent groups [46, 47]. Heterozygous PpargP465L/+ mice have normal total adipose tissue weight, but exhibit reduced intra-abdominal fat mass and increased extra-abdominal subcutaneous fat compared to wild-type (WT) animals, that is, altered body fat distribution, but in a manner which is quite distinct from that observed in human subjects. In addition, unlike their human counterparts, the PpargP465L/+ mice were also insulin-sensitive. These findings initially raised concerns as to the suitability of using rodent models to explore the consequences of loss-of-function mutations in human PPARγ. Importantly however, in the model of Gray et al., expression of the P465L mutant on a hyperphagic ob/ob background grossly exacerbated the insulin resistance and metabolic disturbances associated with leptin deficiency, despite reducing whole body adiposity and adipocyte size [47]. Thus, in the mouse coexistence of the P465L PPARγ mutation and the leptin-deficient state creates a mismatch between adipose tissue expandability and energy availability, thereby unmasking the deleterious effects of PPARγ mutations on carbohydrate metabolism and recapitulating the clinical phenotype observed in human subjects.
3.1.2. Polymorphisms
The most prevalent human PPARγ genetic variant reported to date is the Pro12Ala polymorphism, substituting alanine for proline at codon 12 in the unique PPARγ2 amino-terminal domain [48]. The allelic frequency of the Ala variant differs quite markedly depending on the study population, ranging from 1% to 23% [49]. In functional assays, Ala12-PPARγ exhibits reduced binding to DNA and modest impairment in target gene transactivation in both the absence and presence of PPARγ agonists [48]. An association with lower BMI in the primary study appeared to suggest a corresponding genotype-phenotype correlation, and led to the hypothesis that improved insulin sensitivity might be accounted for entirely by changes in adiposity [48]. However, numerous subsequent cross-sectional studies have yielded conflicting results, demonstrating either no difference [50] or a modestly greater BMI [51] in carriers of the Ala allele. In an attempt to resolve this issue, Masud and Ye completed a meta-analysis using data from 30 independent studies with a total of 19 136 subjects [52]. They concluded that in the samples with a mean BMI value ≥27 kg/m2, Ala12 allele carriers had a significantly higher BMI than noncarriers, whereas no difference was detected in the samples with a BMI value <27 kg/m2. A further analysis using data from publications in which BMI for the three genotype groups (i.e., Pro/Pro, Pro/Ala and Ala/Ala) were presented separately revealed that the Ala12 homozygotes had significantly higher BMI than heterozygotes and Pro12 homozygotes [52].
Importantly, the effects of the Ala allele have recently been shown to be subject to modification by other genetic and environmental factors, and indeed this may in part explain the apparently discordant results of the studies reported hitherto. For example, variations in dietary polyunsaturated fat versus saturated fat intake appear to influence BMI in carriers of the Ala variant [53]. In the Quebec Family Study, carriers of the Pro12 allele had lower BMI, waist circumference and fat mass (both subcutaneous and visceral) at baseline, but responded to an increase in dietary fat with a gradual increase in BMI and waist circumference, an effect which was not observed in their Ala counterparts [54]. Together, these and other studies support the notion of gene-nutrient interaction at the PPARγ locus.
3.2. PPARγ and insulin sensitivity
3.2.1. Genetic evidence for a link
Several lines of evidence point to a link between the level of PPARγ transcriptional activity and insulin sensitivity: (1) the in vitro binding affinities of TZD and non-TZD PPARγ ligands correlate closely with their in vivo potencies as insulin sensitizers [11, 55]; (2) RXR ligands, which can activate the PPARγ-RXR heterodimer, also exhibit insulin-sensitizing effects in rodents [56]; (3) mice exhibiting enhanced PPARγ activity, due to a mutation at serine 112 (serine 114 in human PPARγ2), which results in a constitutively more active receptor (through inhibition of phosphorylation), are protected from obesity-associated insulin resistance [57]; (4) mice lacking PPARγ in fat, muscle, or liver are predisposed to developing insulin resistance [58–61].
Importantly, studies of human PPARγ genetic variants have provided independent validation of the pharmacological and animal data. For example, severe insulin resistance (with or without overt T2DM) has proved to be a remarkably consistent finding in subjects with loss-of-function PPARγ mutations, being evident even in early childhood in affected individuals (Figure 2) [16–23]. Equally impressive has been the finding that of more than 40 different reported associations of genetic variation and population risk to T2DM, Pro12Ala has emerged as the most widely reproduced [62]. The Ala allele is protective against the risk of developing T2DM, and it has been estimated that the global prevalence of T2DM would be ∼25% lower simply by virtue of everybody carrying one or more copies of the Ala allele [49, 62, 63], implying that PPARγ is perhaps the single most important “diabetogene” identified to date.
In light of the findings with Pro12Ala, several groups have sought to determine whether other single-nucleotide polymorphisms (SNPs) within PPARγ might also influence T2DM risk at a population level. In a study of ∼4000 Asian subjects, a link with a second polymorphism C1431T (for which the presence of a T allele conferred a reduced diabetes risk when compared with CC homozygotes (OR = 0.73, P = .011)) has been reported [64]. Other workers have taken analysis of this genetic variant further, establishing it to be in tight allelic disequilibrium with the Ala12 variant in a separate study population (70% of all Ala carriers also carried the C1431T polymorphism) [65]. Having genotyped individuals from three separate cohorts (1997 subjects with T2DM, 2444 nondiabetic children, and 1061 middle-aged controls—all from a similar area in Tayside, Scotland) for the PPARG Pro12Ala and C1431T polymorphisms, they concluded that the Ala12 variant was underrepresented in the T2DM population when compared with similarly aged nondiabetic adults (OR = 0.74, P = .0006). The 1431T variant was also underrepresented in the T2DM versus adult population. Intriguingly however, when the Ala12 variant was on a haplotype not bearing the 1431T variant, it conferred greater protection (OR = 0.66, P = .003); in contrast, when it was present in haplotypes containing the 1431T variant (70% of Ala12 carriers), this protection was absent (OR = 0.99, P = .94). Further studies are awaited with interest.
Thus, it is clear that the relationship between PPARγ activity and insulin sensitivity in humans is complex, with evidence for a gene dosage effect, which is subject to modification by other genetic and environmental factors.
3.2.2. Mechanisms of action
Adipose tissue
Given its high level of expression in adipose tissue and its pivotal role in adipogenesis, it is likely that receptor activation in adipocytes contributes significantly to the clinical efficacy of PPARγ ligands in ameliorating insulin resistance. Consistent with this, mice lacking adipose tissue have been shown to be refractory to the antidiabetic effects of TZDs [66], while adipose-specific deletion of PPARγ (which is associated with progressive lipodystrophy) predisposes mice to hepatic steatosis, and high-fat feeding-induced skeletal muscle insulin resistance [58]. In addition, because PPARγ2 is virtually exclusively expressed in fat cells, any metabolic effects of the Pro12Ala polymorphism, including those on glucose homeostasis, are likely to be secondary to alterations in adipose tissue metabolism. Several mechanisms have been advanced to explain how modulating PPARγ activity in fat benefits whole-body insulin sensitivity.
(i) Regulation of free fatty acid flux in adipocytes
Circulating levels of free fatty acids (FFAs) are a major determinant of insulin sensitivity [38]. Several studies have shown that the antidiabetic efficacy of TZDs correlates with their ability to lower circulating FFA levels [38]. Murine and cellular studies indicate that PPARγ activation in adipose tissue may exert coordinated effects on FFA flux (promoting uptake/trapping, while simultaneously impairing release), through the regulation of a panel of genes involved in FFA metabolism: adipocyte lipoprotein lipase (LPL) expression is upregulated in response to TZD treatment, thereby potentially enhancing release of FFAs from circulating lipoproteins [67]; simultaneous upregulation of FFA transporters such as CD36 and FATP (fatty acid transport protein) on the adipocyte surface facilitates their uptake [68]; TZDs may also reduce FFA efflux from adipocytes through enhanced expression of genes that promote their storage in the form of triglycerides (e.g., glycerol kinase directs the synthesis of glycerol-3-phosphate directly from glycerol; phosphoenolpyruvate carboxykinase permits the utilization of pyruvate to form the glycerol backbone for triglyceride synthesis) [69, 70]. If similar effects on FFA uptake and trapping are observed in human adipocytes, then treatment with TZDs and other PPARγ activators is likely to promote the safe storage of FFAs in adipose tissue, and prevent “ectopic” deposition in other sites such as liver and skeletal muscle, where they are capable of inducing “lipotoxicity.” Observations in human subjects with genetic variations in PPARγ are consistent with this hypothesis. For example, it appears that even the existing residual adipose tissue depots in individuals with loss-of-function mutations in PPARγ are dysfunctional, resulting in exposure of skeletal muscle and liver to unregulated fatty acid fluxes, with consequent impairment of insulin action at these sites [19]. In addition, there is evidence that the Pro12Ala polymorphism facilitates insulin-mediated suppression of lipolysis, hence decreasing FFA release [49]. It is worth noting however that others have failed to detect any relationship between circulating FFA levels and Pro12Ala status [71].
(ii) Modulation of adipokine release
In addition to regulating circulating FFA levels, adipocytes also serve as a rich source of signalling molecules (e.g., leptin, adiponectin, tumour necrosis factor-α (TNFα), and resistin), many of which have far-reaching metabolic effects in other tissues. Collectively these adipocyte-derived hormones are referred to as adipokines, and several have been identified as targets for transcriptional regulation by PPARγ. In general, TZDs and other PPARγ agonists enhance the expression of adipokines that facilitate insulin action while simultaneously suppressing those which are antagonistic, thereby altering the profile of adipocyte gene expression in a manner that promotes insulin sensitization. For example, activation of PPARγ inhibits the expression of TNFα, resistin, and retinol-binding protein 4 (RBP4), all of which are associated with insulin resistance [72–74]. In contrast, adiponectin gene expression is increased following TZD treatment, thereby promoting fatty acid oxidation and insulin sensitivity in muscle and liver [75]. Circulating adiponectin levels have been shown to correlate closely with insulin sensitivity, and inversely with fat mass (especially visceral adiposity) [76], suggesting that this adipokine may represent a critical link between PPARγ activation and insulin sensitization [75, 76]. Consonant with this, circulating adiponectin levels have been shown to be dramatically reduced in individuals harboring loss-of-function PPARγ mutations when compared with healthy controls [77, 78]. In contrast, to date, no definitive correlation between the Pro12Ala polymorphism and adipokine release has been established, with existing studies providing conflicting results.
(iii) Promotion of glucose uptake into adipocytes
There is evidence to suggest that PPARγ is also capable of directly modulating the insulin signal transduction pathway in adipose tissue. The GLUT4 (insulin-dependent) transporter is a key modulator of glucose disposal in both muscle and fat. Binding of insulin to its tyrosine kinase receptor engages a cascade of intracellular phosphorylation events, including activation of phosphatidylinositol-3-OH kinase (PI(3)K) and other downstream kinases, which promote trafficking of GLUT4 containing vesicles to the plasma membrane. A second pathway, which involves a distinct group of signalling molecules including the c-Cbl protooncogene product and CAP (c-Cbl-associated protein), acts in concert to augment this process. Several groups have shown that PPARγ activation in adipose tissue can influence insulin signalling at various points in these pathways, for example, through upregulation of insulin receptor substrates-1 and -2 (IRS-1, IRS-2) [79, 80], the p85 subunit of PI(3)K [81], and CAP [82, 83]—all of which might be predicted to enhance GLUT4 activity. Increased glucose uptake into adipocytes contributes to whole-body glucose disposal, and provides important substrate for triglyceride synthesis.
(iv) Regulation of adipocyte 11β-hydroxysteroid dehydrogenase type 1 activity
Prolonged exposure to hypercortisolaemia, as occurs in subjects with Cushing's syndrome, is associated with many features of the metabolic syndrome (visceral obesity, glucose intolerance, hypertension, and dyslipidaemia). While circulating cortisol levels in ordinary obese non-Cushingoid individuals are normal (if not slightly reduced), there is evidence to suggest that local regeneration of cortisol within adipose tissue could contribute to the development of insulin resistance in the setting of visceral obesity [84]. 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) directs the production of active cortisol from inactive cortisone in liver and fat, thereby facilitating cortisol-induced adipocyte differentiation. In keeping with this, adipose-specific overexpression of 11β-HSD1 in transgenic mice induced a phenotype of insulin resistance and central obesity [85]. PPARγ ligands have been shown to downregulate adipocyte 11β-HSD1 expression and activity [86], and the subsequent modulation of glucocorticoid-induced gene expression may conceivably contribute to their insulin sensitizing actions. Studies of 11β-HSD1 activity in adipose tissue from subjects with loss-of-function mutations in PPARγ should provide a unique opportunity to examine the role of PPARγ in regulating human 11β-HSD1 function.
Skeletal muscle and liver
Maintenance of normal glucose homeostasis is critically dependent on retention of insulin sensitivity in key target tissues including liver and skeletal muscle. In addition to the beneficial effects of lowering circulating FFA levels and inducing a more favorable adipokine milieu to promote insulin sensitivity, there is some evidence to suggest that PPARγ activation at both of these sites might directly influence glucose and lipid homeostasis. For example, TZDs have been reported to facilitate insulin-stimulated glucose uptake in cultured human skeletal muscle cells, by enhancing insulin-stimulated PI(3)K activity and GLUT4 translocation [87, 88]. Thus, while skeletal muscle expresses relatively low levels of PPARγ protein when compared with adipose tissue, its dominant role in insulin-mediated glucose disposal suggests that PPARγ activation at this site may contribute significantly to the glucose lowering effect of TZD treatment. Unfortunately, to date attempts to resolve this issue using animal models of muscle-specific PPARγ deletion have proved unsuccessful with two separate groups reporting conflicting findings [59, 60]. Similarly, it remains to be seen whether activation of PPARγ in human liver benefits or impairs metabolic function, with further studies needed to clarify its role in the regulation of hepatic gluconeogenesis and susceptibility to hepatic steatosis.
3.3. PPARγ and lipid homeostasis
As might be predicted for a group of drugs that improve insulin sensitivity, TZDs raise HDL cholesterol in the majority of treated diabetics (typically by 5%–10% ) [8]. Intriguingly however, their effects on hypertriglyceridaemia have been somewhat more variable, with reductions in triglyceride levels observed more often with pioglitazone than rosiglitazone. One hypothesis that has been advanced to explain this apparent discrepancy is that pioglitazone may also be acting as a partial PPARα agonist (akin to a fibrate), while at the doses used in clinical practice rosiglitazone retains pure γ-agonist activity [89]. However, data on mechanisms underlying the effects of TZDs on lipids in humans is limited and, moreover, caution needs to be exercised when attempting to extrapolate from animal studies, given the significant species-specific differences that exist in lipoprotein metabolism.
To date, virtually all subjects with loss-of-function mutations in PPARγ have exhibited hypertriglyceridaemia and/or low HDL levels, with relatively unremarkable LDL cholesterols [16–23] (Figure 2). It remains unclear however, as to whether these abnormalities are simply a “metabolic consequence” of severe insulin resistance per se, or whether they indicate an additive and independent effect of dysfunctional PPARγ signalling in relation to lipoprotein metabolism. Further studies of the reverse cholesterol transport pathway in monocyte-derived macrophages from these subjects may help to address this important issue.
Although there is an extensive body of data concerning the potential effects of the Pro12Ala polymorphism on glycaemic control, there are relatively few studies focusing on its consequences for lipid homeostasis. Moreover, given the potential confounding effect of insulin resistance, cohort selection (particularly with respect to diabetic status and/or BMI) is critical when trying to identify a specific independent link. Accepting these limitations, there is some evidence to suggest that the Ala allele may confer benefits for HDL metabolism. For example, in the original study of Deeb et al., higher HDL cholesterol (and lower triglyceride) levels were observed among elderly subjects with the Ala/Ala genotype compared with Pro/Ala and Pro/Pro genotypes [48]. A similar association has been described in over 4000 Singapore Asians whose genotype was analyzed as a dichotomous variable (i.e., presence or absence of the Ala variant), and in whom Ala allele carriers had significantly higher HDL cholesterol compared with Pro/Pro homozygotes [64]. However, other groups have reported conflicting findings, with some detecting an association of lower HDL cholesterol levels with the presence of the Ala allele [50].
3.4. PPARγ and blood pressure regulation
Hypertension has been reported in a significant proportion of subjects harboring PPARγ mutations [16–23]. While this is not unexpected, given the well-recognized associations of insulin resistance and T2DM with hypertension, it is noteworthy that in some cases the hypertension has been of an unusually early onset and severity [16, 19, 23]. Indeed, on occasion it has been the dominant clinical feature, manifesting even in the absence of diabetes and its associated microvascular complications. In contrast, TZD therapy is associated with a modest reduction in blood pressure in a variety of clinical settings, including nondiabetic hypertensive subjects [89]. Taken together, these findings suggest possible additional effects on blood pressure regulation, which are independent of insulin sensitivity, and indeed several lines of evidence suggest that PPARγ may directly regulate vascular tone, for example, through blockade of calcium channel activity in smooth muscle, inhibition of release of endothelin-1, and enhancement of C-type natriuretic peptide release [89].
While no studies of vascular tone or endothelial function have yet been reported in human subjects with PPARγ mutations, mice heterozygous for the equivalent Pro465Leu mutation were found to be hypertensive in the absence of insulin resistance [46]. The hypertension in PpargP465L/+ mice was associated with increased expression of RAS components in various adipose depots—angiotensinogen (AGT) and angiotensin II receptor subtype 1 (AT1R) in inguinal and gonadal fat, respectively [46]. Interestingly, transgenic mice expressing AGT in adipose tissue have higher BP and increased fat mass [90]. Thus, it is conceivable that modulation of RAS activity in adipose tissue contributes to the decrease in blood pressure, which is seen with TZDs and other PPARγ agonists.
Data relating to differences in blood pressure and Pro12Ala status have proved less informative, with studies again reporting conflicting findings [91, 92], which are likely to reflect other genetic and environmental influences that are at work in the different study populations.
3.5. PPARγ and atherosclerosis
Collectively, the individual components of the metabolic syndrome conspire to dramatically increase the risk of cardiovascular disease [93]. PPARγ activation with exogenous ligands such as the TZDs would be predicted to confer significant benefits in this setting, through the amelioration of insulin resistance, dyslipidaemia, and possibly hypertension, albeit at a potential cost of mild weight gain (as a consequence of enhanced adipogenesis and fluid retention). Indeed retrospective human studies have indirectly suggested an atheroprotective effect of TZDs [94], and more recently a prospective trial demonstrated that pioglitazone protected patients with T2DM, albeit modestly, from cardiovascular events [9].
It was therefore surprising and of potential therapeutic concern when Tontonoz et al. reported that PPARγ activation in a premacrophage cell line induced expression of CD36 (also known as FAT—fatty acid translocase), a cellular scavenger receptor for atherogenic low-density lipoprotein (LDL) [95]. Enhanced CD36 expression might be predicted to increase intracellular accumulation of oxidized LDL cholesterol, which could then be catabolized to generate PPARγ ligands (e.g., 9-hydroxyoctadecadienoic acid (9-HODE) and 13-HODE) capable of further receptor activation, thereby creating a vicious feedforward cycle of increasing lipid uptake, and ultimately driving conversion of the macrophage into an atherogenic foam cell [95, 96]. The finding that PPARγ is expressed at relatively high levels in human atherosclerotic plaques further served to fuel concerns [97].
However, almost coincident with these observations, several groups reported that PPARγ ligands could reduce the release of inflammatory cytokines (e.g., TNF-α and IL-6) from macrophages, an effect that might be predicted to be antiatherogenic [98, 99]. Several anti-inflammatory mechanisms have been proposed, including inhibition of NF-kB, AP1, and STAT signalling by PPARγ [100].
Subsequent studies have further redressed the balance, with the demonstration that PPARγ ligands exert an opposing effect on SR-A, a second LDL scavenger receptor, downregulating its expression in mouse macrophages [101]. In addition, the nuclear receptor LXRα (liver X receptor α), which enhances expression of ABCA1 (ATP-binding cassette transporter A1), a protein which mediates cellular cholesterol efflux [102], has also been shown to be a PPARγ target gene in human and mouse macrophages [103, 104]. Taken together, these data suggest a broader spectrum of PPARγ effects within the macrophage with the overall balance favouring cholesterol efflux and an antiatherogenic effect.
At first glance, the finding that only six subjects from a cohort of more than 20 affected PPARγ mutation carriers [18, 23] have documented atheromatous coronary disease might seem surprisingly modest, especially when one considers the severity of insulin resistance, dyslipidaemia, and hypertension found in this group, coupled with the potentially deleterious consequences of dysfunctional PPARγ signalling inside mutant macrophages. However, it is important to note that four of the six affected subjects are/were relatively young females in whom atheromatous coronary disease in the general population is a relatively uncommon occurrence. Accordingly, given that many of the remaining mutation carriers are still relatively young (<50 years), with a predominance of females, it would seem premature to exclude the possibility of accelerated vascular disease in this high-risk group.
There is also an emerging body of epidemiological evidence to suggest an association between the naturally occurring PPARγ polymorphisms and arterial intima media thickness (IMT), and thus indirectly, cardiovascular risk. A study of 154 Japanese T2DM patients found those carrying the Ala12 allele to have a significantly lower carotid IMT than their Pro/Pro counterparts, despite no observed differences in gender, age, fasting blood glucose, lipid profile, or HbA1c [105]. However, differences in BMI and the degree of insulin resistance between the two groups were not reported. Yan et al. used IMT as a secondary outcome measure to investigate the prevalence of the C161T PPARγ polymorphism within 4 different Chinese cohorts; 248 subjects with insulin resistance syndrome (IRS), 163 with essential hypertension, 115 with T2DM and 121 normal controls. They observed that the CC genotype (prevalence 75%) was significantly associated with increased IMT compared to CT and TT genotypes (prevalence 22% and 4%, resp.) within 248 “metabolic syndrome” patients [106]. However interestingly, the prevalence of neither the Pro12Ala nor C161T polymorphism within PPARγ was overrepresented in a large Caucasian cohort (1170 individuals) with angiographically proven coronary heart disease [50], and it is clear that further large-scale studies are needed.
4. SELECTIVE PPARγ MODULATION
The ability of TZDs such as rosiglitazone and pioglitazone to enhance insulin sensitivity makes them attractive agents for use in the treatment of T2DM and the metabolic syndrome. Unfortunately however, the initial excitement that followed the introduction of TZDs into clinical practice has been tempered by the realization that for many patients, they afford only modest benefits in terms of glycaemic control—typically lowering glycosylated haemoglobin levels by 1.0%–1.5%—at a cost of weight gain and, in some instances, fluid retention/peripheral oedema [8]. Nevertheless, they represent a “step in the right direction” and have served to emphasize the potential benefits and limitations of modulating PPARγ function in human subjects.
For those seeking to develop the next generation of PPARγ ligands, two (related) key questions must be answered: (1) how much PPARγ activation is desirable, (2) is it possible to separate the receptor's adipogenic actions from those mediating improved insulin sensitivity, that is, to develop selective receptor modulators (so-called SPPARMs) that are capable of regulating glucose and lipid metabolism without promoting adipogenesis. Taking this a step further, if such agents favourably altered receptor function at other sites, for example, within macrophages and the vasculature, then it is conceivable that we might have access to a class of drugs which is almost tailor-made for treating the metabolic syndrome. Precedent for such an approach is provided by raloxifene, a selective oestrogen receptor (ER) modulator (SERM), which is an ER antagonist in breast and endometrium, but an agonist in bone. Examination of the properties of PPARγ in adipocytes suggests that it may be possible to selectively modulate its function in an analogous manner. For example, inside mature adipocytes, certain PPARγ target genes, for example, GyK, require exogenous ligand for activation, while others, for example, aP2, are activated even in the absence of synthetic ligand [24]. The concept of differential modulation of PPARγ activity is also supported by the work of Li and Lazar who have demonstrated that a form of this protein rendered constitutively active by fusion to the powerful VP16 transactivation domain could switch on the adipogenic gene program, yet it was unable to transrepress other PPAR target genes such as that encoding resistin [107].
Promisingly, several groups have independently identified PPARγ ligands with partial agonist activity and only mild/modest effects on adipogenesis, yet with retention of insulin sensitizing properties. MCC-555 is one such compound, whose ability to stimulate PPARγ is highly context-specific [108]. FMOC-L-Leucine, a chemically distinct receptor ligand, whose gene-specific effects appear to reflect differential coactivator recruitment, has been shown to improve insulin sensitivity, yet exert relatively weak adipogenic effects in rodent diabetic models [109]. Similarly, YM440, an analog of the oxadiazolidinediones, improved glycaemic control, but did not alter body fat weight in diabetic db/db mice [110].
The discovery of such compounds has prompted widespread screening of libraries of both structurally related and chemically distinct molecules with the subsequent identification of an array of potential SPPARMs: PAT5a, an unsaturated TZD with partial agonist activity, is a potent antidiabetic agent with only weak adipogenic activity [111]; similar properties have been reported for the novel non-TZD-selective PPARγ modulators nTZDpa [112] and KR-62980 [113]; a panel of N-benzyl-indole-selective PPARγ modulators, with partial agonist activity in vitro, exhibited potent glucose-lowering activity in db/db mice, but attenuated increases in heart weight and brown adipose tissue when compared with full agonists [114]. Interestingly, the message that seems to be emerging from these and other similar studies is that ‘activation in moderation’ is the way forward for PPARγ, thus confirming the adage that you can indeed have ‘too much of a good thing.’
5. CONCLUSIONS
In just over a decade, PPARγ has evolved from modest beginnings as a simple regulator of adipogenesis to become a key therapeutic target in the fight against the 21st century epidemics of obesity, insulin resistance, and the metabolic syndrome. While pharmacological and animal studies have yielded important information regarding the role of this receptor in the regulation of energy, glucose, and lipid homeostasis, there is little doubt that defining the metabolic consequences associated with polymorphisms and mutations in the human PPARγ gene has contributed significantly to our understanding of the biology of this receptor. Given the significant species-specific differences that exist in metabolism, particularly in relation to lipid homeostasis, it is critical that we continue to identify and study these human experiments of nature, in order to complement the impressive pharmacological and functional genomic approaches that are currently being used to facilitate the development of more superior ligands with enhanced therapeutic impact. Given the apparent inexorable rise in the prevalence of obesity, insulin resistance, and T2DM, the need for such novel therapies could not be more urgent.
ACKNOWLEDGMENTS
The author would like to acknowledge the support of the Wellcome Trust, and the clinicians and colleagues who have referred cases for study and contributed to the laboratory and physiological studies reported herein.
References
- 1.Olshansky SJ, Passaro DJ, Hershow RC, et al. A potential decline in life expectancy in the United States in the 21st century. New England Journal of Medicine. 2005;352(11):1138–1145. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. Journal of the American Medical Association. 2006;295(13):1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 3.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. The Lancet. 2005;365(9468):1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 4.Tuomilehto J, Lindström J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. New England Journal of Medicine. 2001;344(18):1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 5.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. New England Journal of Medicine. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Auwerx J. PPARγ, the ultimate thrifty gene. Diabetologia. 1999;42(9):1033–1049. doi: 10.1007/s001250051268. [DOI] [PubMed] [Google Scholar]
- 7.Forman BM, Chen J, Evans RM. The peroxisome proliferator-activated receptors: ligands and activators. Annals of the New York Academy of Sciences. 1996;804:266–275. doi: 10.1111/j.1749-6632.1996.tb18621.x. [DOI] [PubMed] [Google Scholar]
- 8.Yki-Järvinen H. Thiazolidinediones. New England Journal of Medicine. 2004;351(11):1106–1118. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]
- 9.Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive study (PROspective pioglitAzone clinical trial in macro vascular events): a randomised controlled trial. The Lancet. 2005;366(9493):1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 10.DREAM trial investigators Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. The Lancet. 2006;368(9541):1096–1105. doi: 10.1016/S0140-6736(06)69420-8. [DOI] [PubMed] [Google Scholar]
- 11.Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor γ (PPARγ) Journal of Biological Chemistry. 1995;270(22):12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 12.Fonseca V. Effect of thiazolidinediones on body weight in patients with diabetes mellitus. American Journal of Medicine. 2003;115(8, supplement 1):42–48. doi: 10.1016/j.amjmed.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Larsen TM, Toubro S, Astrup A. PPARγ agonists in the treatment of type II diabetes: is increased fatness commensurate with long-term efficacy? International Journal of Obesity. 2003;27(2):147–161. doi: 10.1038/sj.ijo.802223. [DOI] [PubMed] [Google Scholar]
- 14.Guan Y, Hao C, Cha DR, et al. Thiazolidinediones expand body fluid volume through PPARγ stimulation of ENaC-mediated renal salt absorption. Nature Medicine. 2005;11(8):861–866. doi: 10.1038/nm1278. [DOI] [PubMed] [Google Scholar]
- 15.Mudaliar S, Chang AR, Henry RR. Thiazolidinediones, peripheral edema, and type 2 diabetes: incidence, pathophysiology, and clinical implications. Endocrine Practice. 2003;9(5):406–416. doi: 10.4158/EP.9.5.406. [DOI] [PubMed] [Google Scholar]
- 16.Barroso I, Gurnell M, Crowley VEF, et al. Dominant negative mutations in human PPARγ associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402(6764):880–883. doi: 10.1038/47254. [DOI] [PubMed] [Google Scholar]
- 17.Agarwal AK, Garg A. A novel heterozygous mutation in peroxisome proliferator-activated receptor-γ gene in a patient with familial partial lipodystrophy. Journal of Clinical Endocrinology and Metabolism. 2002;87(1):408–411. doi: 10.1210/jcem.87.1.8290. [DOI] [PubMed] [Google Scholar]
- 18.Hegele RA, Cao H, Frankowski C, Mathews ST, Leff T. PPARG F388L, a transactivation-deficient mutant, in familial partial lipodystrophy. Diabetes. 2002;51(12):3586–3590. doi: 10.2337/diabetes.51.12.3586. [DOI] [PubMed] [Google Scholar]
- 19.Savage DB, Tan GD, Acerini CL, et al. Human metabolic syndrome resulting from dominant-negative mutations in the nuclear receptor peroxisome proliferator-activated receptor-γ . Diabetes. 2003;52(4):910–917. doi: 10.2337/diabetes.52.4.910. [DOI] [PubMed] [Google Scholar]
- 20.Savage DB, Agostini M, Barroso I, et al. Digenic inheritance of severe insulin resistance in a human pedigree. Nature Genetics. 2002;31(4):379–384. doi: 10.1038/ng926. [DOI] [PubMed] [Google Scholar]
- 21.Francis GA, Li G, Casey R, et al. Peroxisomal proliferator activated receptor-γ deficiency in a Canadian kindred with familial partial lipodystrophy type 3 (FPLD3) BMC Medical Genetics. 2006;7:3. doi: 10.1186/1471-2350-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hegele RA, Ur E, Ransom TP, Cao H. A frameshift mutation in peroxisome-proliferator-activated receptor-γ in familial partial lipodystrophy subtype 3 (FPLD3; MIM 604367) Clinical Genetics. 2006;70(4):360–362. doi: 10.1111/j.1399-0004.2006.00674.x. [DOI] [PubMed] [Google Scholar]
- 23.Agostini M, Schoenmakers E, Mitchell C, et al. Non-DNA binding, dominant-negative, human PPARγ mutations cause lipodystrophic insulin resistance. Cell Metabolism. 2006;4(4):303–311. doi: 10.1016/j.cmet.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guan H-P, Ishizuka T, Chui PC, Lehrke M, Lazar MA. Corepressors selectively control the transcriptional activity of PPARγ in adipocytes. Genes and Development. 2005;19(4):453–461. doi: 10.1101/gad.1263305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans RM, Barish GD, Wang Y-X. PPARs and the complex journey to obesity. Nature Medicine. 2004;10(4):355–361. doi: 10.1038/nm1025. [DOI] [PubMed] [Google Scholar]
- 26.Barish GD, Narkar VA, Evans RM. PPARδ: a dagger in the heart of the metabolic syndrome. Journal of Clinical Investigation. 2006;116(3):590–597. doi: 10.1172/JCI27955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fajas L, Auboeuf D, Raspé E, et al. The organization, promoter analysis, and expression of the human PPARγ gene. Journal of Biological Chemistry. 1997;272(30):18779–18789. doi: 10.1074/jbc.272.30.18779. [DOI] [PubMed] [Google Scholar]
- 28.Fajas L, Fruchart J-C, Auwerx J. PPARγ3 mRNA: a distinct PPARγ mRNA subtype transcribed from an independent promoter. FEBS Letters. 1998;438(1-2):55–60. doi: 10.1016/s0014-5793(98)01273-3. [DOI] [PubMed] [Google Scholar]
- 29.Sundvold H, Lien S. Identification of a novel peroxisome proliferator-activated receptor (PPAR) γ promoter in man and transactivation by the nuclear receptor RORα1. Biochemical and Biophysical Research Communications. 2001;287(2):383–390. doi: 10.1006/bbrc.2001.5602. [DOI] [PubMed] [Google Scholar]
- 30.Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocrine Reviews. 1999;20(5):649–688. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- 31.Tzameli I, Fang H, Ollero M, et al. Regulated production of a peroxisome proliferator-activated receptor-γ ligand during an early phase of adipocyte differentiation in 3T3-L1 adipocytes. Journal of Biological Chemistry. 2004;279(34):36093–36102. doi: 10.1074/jbc.M405346200. [DOI] [PubMed] [Google Scholar]
- 32.Rosen ED, Hsu C-H, Wang X, et al. C/EBPα induces adipogenesis through PPARγ: a unified pathway. Genes and Development. 2002;16(1):22–26. doi: 10.1101/gad.948702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid-activated transcription factor. Cell. 1994;79(7):1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 34.Barak Y, Nelson MC, Ong ES, et al. PPARγ is required for placental, cardiac, and adipose tissue development. Molecular Cell. 1999;4(4):585–595. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- 35.Rosen ED, Sarraf P, Troy AE, et al. PPARγ is required for the differentiation of adipose tissue in vivo and in vitro. Molecular Cell. 1999;4(4):611–617. doi: 10.1016/s1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- 36.Kubota N, Terauchi Y, Miki H, et al. PPARγ mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Molecular Cell. 1999;4(4):597–609. doi: 10.1016/s1097-2765(00)80210-5. [DOI] [PubMed] [Google Scholar]
- 37.Gurnell M, Wentworth JM, Agostini M, et al. A dominant-negative peroxisome proliferator-activated receptor γ (PPARγ) mutant is a constitutive repressor and inhibits PPARγ-mediated adipogenesis. Journal of Biological Chemistry. 2000;275(8):5754–5759. doi: 10.1074/jbc.275.8.5754. [DOI] [PubMed] [Google Scholar]
- 38.Bays H, Mandarino L, DeFronzo RA. Role of the adipocyte, free fatty acids, and ectopic fat in pathogenesis of type 2 diabetes mellitus: peroxisomal proliferator-activated receptor agonists provide a rational therapeutic approach. Journal of Clinical Endocrinology and Metabolism. 2004;89(2):463–478. doi: 10.1210/jc.2003-030723. [DOI] [PubMed] [Google Scholar]
- 39.Adams M, Montague CT, Prins JB, et al. Activators of peroxisome proliferator-activated receptor γ have depot-specific effects on human preadipocyte differentiation. Journal of Clinical Investigation. 1997;100(12):3149–3153. doi: 10.1172/JCI119870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ristow M, Müller-Wieland D, Pfeiffer A, Krone W, Kahn CR. Obesity associated with a mutation in a genetic regulator of adipocyte differentiation. New England Journal of Medicine. 1998;339(14):953–959. doi: 10.1056/NEJM199810013391403. [DOI] [PubMed] [Google Scholar]
- 41.Hu E, Kim JB, Sarraf P, Spiegelman BM. Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARγ . Science. 1996;274(5295):2100–2103. doi: 10.1126/science.274.5295.2100. [DOI] [PubMed] [Google Scholar]
- 42.Adams M, Reginato MJ, Shao D, Lazar MA, Chatterjee VK. Transcriptional activation by peroxisome proliferator-activated receptor γ is inhibited by phosphorylation at a consensus mitogen-activated protein kinase site. Journal of Biological Chemistry. 1997;272(8):5128–5132. doi: 10.1074/jbc.272.8.5128. [DOI] [PubMed] [Google Scholar]
- 43.Blüher M, Paschke R. Analysis of the relationship between PPAR-γ 2 gene variants and severe insulin resistance in obese patients with impaired glucose tolerance. Experimental and Clinical Endocrinology and Diabetes. 2003;111(2):85–90. doi: 10.1055/s-2003-39235. [DOI] [PubMed] [Google Scholar]
- 44.Agostini M, Gurnell M, Savage DB, et al. Tyrosine agonists reverse the molecular defects associated with dominant-negative mutations in human peroxisome proliferator-activated receptor γ . Endocrinology. 2004;145(4):1527–1538. doi: 10.1210/en.2003-1271. [DOI] [PubMed] [Google Scholar]
- 45.Al-Shali K, Cao H, Knoers N, Hermus AR, Tack CJ, Hegele RA. A single-base mutation in the peroxisome proliferator-activated receptor γ4 promoter associated with altered in vitro expression and partial lipodystrophy. Journal of Clinical Endocrinology and Metabolism. 2004;89(11):5655–5660. doi: 10.1210/jc.2004-0280. [DOI] [PubMed] [Google Scholar]
- 46.Tsai Y-S, Kim H-J, Takahashi N, et al. Hypertension and abnormal fat distribution but not insulin resistance in mice with P465L PPARγ . Journal of Clinical Investigation. 2004;114(2):240–249. doi: 10.1172/JCI20964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gray SL, Nora ED, Grosse J, et al. Leptin deficiency unmasks the deleterious effects of impaired peroxisome proliferator-activated receptor γ function (P465L PPARγ) in mice. Diabetes. 2006;55(10):2669–2677. doi: 10.2337/db06-0389. [DOI] [PubMed] [Google Scholar]
- 48.Deeb SS, Fajas L, Nemoto M, et al. A Pro12Ala substitution in PPARγ2 associated with decreased receptor activity, lower body mass index and improved insulin sensitivity. Nature Genetics. 1998;20(3):284–287. doi: 10.1038/3099. [DOI] [PubMed] [Google Scholar]
- 49.Stumvoll M, Häring H. The peroxisome proliferator-activated receptor-γ2 Pro12Ala polymorphism. Diabetes. 2002;51(8):2341–2347. doi: 10.2337/diabetes.51.8.2341. [DOI] [PubMed] [Google Scholar]
- 50.Swarbrick MM, Chapman CML, McQuillan BM, Hung J, Thompson PL, Beilby JP. A Pro12Ala polymorphism in the human peroxisome proliferator-activated receptor-γ2 is associated with combined hyperlipidaemia in obesity. European Journal of Endocrinology. 2001;144(3):277–282. doi: 10.1530/eje.0.1440277. [DOI] [PubMed] [Google Scholar]
- 51.Beamer BA, Yen C-J, Andersen RE, et al. Association of the Pro12Ala variant in the peroxisome proliferator-activated receptor-γ2 gene with obesity in two Caucasian populations. Diabetes. 1998;47(11):1806–1808. doi: 10.2337/diabetes.47.11.1806. [DOI] [PubMed] [Google Scholar]
- 52.Masud S, Ye S. Effect of the peroxisome proliferates activated receptor-γ gene Pro12Ala variant on body mass index: a meta-analysis. Journal of Medical Genetics. 2003;40(10):773–780. doi: 10.1136/jmg.40.10.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luan J, Browne PO, Harding A-H, et al. Evidence for gene-nutrient interaction at the PPARγ locus. Diabetes. 2001;50(3):686–689. doi: 10.2337/diabetes.50.3.686. [DOI] [PubMed] [Google Scholar]
- 54.Robitaille J, Després J-P, Pérusse L, Vohl M-C. The PPAR-γ P12A polymorphism modulates the relationship between dietary fat intake and components of the metabolic syndrome: results from the Québec family study. Clinical Genetics. 2003;63(2):109–116. doi: 10.1034/j.1399-0004.2003.00026.x. [DOI] [PubMed] [Google Scholar]
- 55.Willson TM, Cobb JE, Cowan DJ, et al. The structure—activity relationship between peroxisome proliferator-activated receptor γ agonism and the antihyperglycemic activity of thiazolidinediones. Journal of Medicinal Chemistry. 1996;39(3):665–668. doi: 10.1021/jm950395a. [DOI] [PubMed] [Google Scholar]
- 56.Mukherjee R, Davies PJA, Crombie DL, et al. Sensitization of diabetic and obese mice to insulin by retinoid X receptor agonists. Nature. 1997;386(6623):407–410. doi: 10.1038/386407a0. [DOI] [PubMed] [Google Scholar]
- 57.Rangwala SM, Rhoades B, Shapiro JS, et al. Genetic modulation of PPARγ phosphorylation regulates insulin sensitivity. Developmental Cell. 2003;5(4):657–663. doi: 10.1016/s1534-5807(03)00274-0. [DOI] [PubMed] [Google Scholar]
- 58.He W, Barak Y, Hevener A, et al. Adipose-specific peroxisome proliferator-activated receptor γ knockout causes insulin resistance in fat and liver but not in muscle. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(26):15712–15717. doi: 10.1073/pnas.2536828100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hevener AL, He W, Barak Y, et al. Muscle-specific Pparg deletion causes insulin resistance. Nature Medicine. 2003;9(12):1491–1497. doi: 10.1038/nm956. [DOI] [PubMed] [Google Scholar]
- 60.Norris AW, Chen L, Fisher SJ, et al. Muscle-specific PPARγ-deficient mice develop increased adiposity and insulin resistance but respond to thiazolidinediones. Journal of Clinical Investigation. 2003;112(4):608–618. doi: 10.1172/JCI17305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matsusue K, Haluzik M, Lambert G, et al. Liver-specific disruption of PPARγ in leptin-deficient mice improves fatty liver but aggravates diabetic phenotypes. Journal of Clinical Investigation. 2003;111(5):737–747. doi: 10.1172/JCI17223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Florez JC, Hirschhorn J, Altshuler D. The inherited basis of diabetes mellitus: implications for the genetic analysis of complex traits. Annual Review of Genomics and Human Genetics. 2003;4:257–291. doi: 10.1146/annurev.genom.4.070802.110436. [DOI] [PubMed] [Google Scholar]
- 63.Memisoglu A, Hu FB, Hankinson SE, et al. Prospective study of the association between the proline to alanine codon 12 polymorphism in the PPARγ gene and type 2 diabetes. Diabetes Care. 2003;26(10):2915–2917. doi: 10.2337/diacare.26.10.2915. [DOI] [PubMed] [Google Scholar]
- 64.Tai ES, Corella D, Deurenberg-Yap M, et al. Differential effects of the C1431T and Pro12Ala PPARγ gene variants on plasma lipids and diabetes risk in an Asian population. Journal of Lipid Research. 2004;45(4):674–685. doi: 10.1194/jlr.M300363-JLR200. [DOI] [PubMed] [Google Scholar]
- 65.Doney ASF, Fischer B, Cecil JE, et al. Association of the Pro12Ala and C1431T variants of PPARG and their haplotypes with susceptibility to type 2 diabetes. Diabetologia. 2004;47(3):555–558. doi: 10.1007/s00125-003-1323-1. [DOI] [PubMed] [Google Scholar]
- 66.Chao L, Marcus-Samuels B, Mason MM, et al. Adipose tissue is required for the antidiabetic, but not for the hypolipidemic, effect of thiazolidinediones. Journal of Clinical Investigation. 2000;106(10):1221–1228. doi: 10.1172/JCI11245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schoonjans K, Peinado-Onsurbe J, Lefebvre A-M, et al. PPARα and PPARγ activators direct a distinct tissue-specific transcriptional response via a PPRE in the lipoprotein lipase gene. EMBO Journal. 1996;15(19):5336–5348. [PMC free article] [PubMed] [Google Scholar]
- 68.Frohnert BI, Hui TY, Bernlohr DA. Identification of a functional peroxisome proliferator-responsive element in the murine fatty acid transport protein gene. Journal of Biological Chemistry. 1999;274(7):3970–3977. doi: 10.1074/jbc.274.7.3970. [DOI] [PubMed] [Google Scholar]
- 69.Guan H-P, Li Y, Jensen MV, Newgard CB, Steppan CM, Lazar MA. A futile metabolic cycle activated in adipocytes by antidiabetic agents. Nature Medicine. 2002;8(10):1122–1128. doi: 10.1038/nm780. [DOI] [PubMed] [Google Scholar]
- 70.Tordjman J, Chauvet G, Quette J, Beale EG, Forest C, Antoine B. Thiazolidinediones block fatty acid release by inducing glyceroneogenesis in fat cells. Journal of Biological Chemistry. 2003;278(21):18785–18790. doi: 10.1074/jbc.M206999200. [DOI] [PubMed] [Google Scholar]
- 71.Vaccaro O, Mancini FP, Ruffa G, et al. Fasting plasma free fatty acid concentrations and Pro12Ala polymorphism of the peroxisome proliferator-activated receptor (PPAR) γ2 gene in healthy individuals. Clinical Endocrinology. 2002;57(4):481–486. doi: 10.1046/j.1365-2265.2002.01618.x. [DOI] [PubMed] [Google Scholar]
- 72.Peraldi P, Xu M, Spiegelman BM. Thiazolidinediones block tumor necrosis factor-α-induced inhibition of insulin signaling. Journal of Clinical Investigation. 1997;100(7):1863–1869. doi: 10.1172/JCI119715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Steppan CM, Bailey ST, Bhat S, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409(6818):307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 74.Yang Q, Graham TE, Mody N, et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436(7049):356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 75.Maeda N, Takahashi M, Funahashi T, et al. PPARγ ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes. 2001;50(9):2094–2099. doi: 10.2337/diabetes.50.9.2094. [DOI] [PubMed] [Google Scholar]
- 76.Ahima RS, Flier JS. Adipose tissue as an endocrine organ. Trends in Endocrinology and Metabolism. 2000;11(8):327–332. doi: 10.1016/s1043-2760(00)00301-5. [DOI] [PubMed] [Google Scholar]
- 77.Combs TP, Wagner JA, Berger J, et al. Induction of adipocyte complement-related protein of 30 kilodaltons by PPARγ agonists: a potential mechanism of insulin sensitization. Endocrinology. 2002;143(3):998–1007. doi: 10.1210/endo.143.3.8662. [DOI] [PubMed] [Google Scholar]
- 78.Semple RK, Soos MA, Luan J, et al. Elevated plasma adiponectin in humans with genetically defective insulin receptors. Journal of Clinical Endocrinology and Metabolism. 2006;91(8):3219–3223. doi: 10.1210/jc.2006-0166. [DOI] [PubMed] [Google Scholar]
- 79.Iwata M, Haruta T, Usui I, et al. Pioglitazone ameliorates tumor necrosis factor-α-induced insulin resistance by a mechanism independent of adipogenic activity of peroxisome proliferator-activated receptor-γ . Diabetes. 2001;50(5):1083–1092. doi: 10.2337/diabetes.50.5.1083. [DOI] [PubMed] [Google Scholar]
- 80.Smith U, Gogg S, Johansson A, Olausson T, Rotter V, Svalstedt B. Thiazolidinediones (PPARγ agonists) but not PPARα agonists increase IRS-2 gene expression in 3T3-L1 and human adipocytes. FASEB Journal. 2001;15(1):215–220. doi: 10.1096/fj.00-0020com. [DOI] [PubMed] [Google Scholar]
- 81.Rieusset J, Chambrier C, Bouzakri K, et al. The expression of the p85α subunit of phosphatidylinositol 3-kinase is induced by activation of the peroxisome proliferator-activated receptor γ in human adipocytes. Diabetologia. 2001;44(5):544–554. doi: 10.1007/s001250051660. [DOI] [PubMed] [Google Scholar]
- 82.Ribon V, Johnson JH, Camp HS, Saltiel AR. Thiazolidinediones and insulin resistance: peroxisome proliferator-activated receptor γ activation stimulates expression of the CAP gene. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(25):14751–14756. doi: 10.1073/pnas.95.25.14751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Baumann CA, Chokshi N, Saltiel AR, Ribon V. Cloning and characterization of a functional peroxisome proliferator activator receptor-γ-responsive element in the promoter of the CAP gene. Journal of Biological Chemistry. 2000;275(13):9131–9135. doi: 10.1074/jbc.275.13.9131. [DOI] [PubMed] [Google Scholar]
- 84.Bujalska IJ, Kumar S, Stewart PM. Does central obesity reflect “Cushing's disease of the omentum”? The Lancet. 1997;349(9060):1210–1213. doi: 10.1016/S0140-6736(96)11222-8. [DOI] [PubMed] [Google Scholar]
- 85.Masuzaki H, Paterson J, Shinyama H, et al. A transgenic model of visceral obesity and the metabolic syndrome. Science. 2001;294(5549):2166–2170. doi: 10.1126/science.1066285. [DOI] [PubMed] [Google Scholar]
- 86.Berger J, Tanen M, Elbrecht A, et al. Peroxisome proliferator-activated receptor-γ ligands inhibit adipocyte 11β-hydroxysteroid dehydrogenase type 1 expression and activity. Journal of Biological Chemistry. 2001;276(16):12629–12635. doi: 10.1074/jbc.M003592200. [DOI] [PubMed] [Google Scholar]
- 87.Cha BS, Ciaraldi TP, Carter L, et al. Peroxisome proliferator-activated receptor (PPAR) γ and retinoid X receptor (RXR) agonists have complementary effects on glucose and lipid metabolism in human skeletal muscle. Diabetologia. 2001;44(4):444–452. doi: 10.1007/s001250051642. [DOI] [PubMed] [Google Scholar]
- 88.Kausch C, Krützfeldt J, Witke A, et al. Effects of troglitazone on cellular differentiation, insulin signaling, and glucose metabolism in cultured human skeletal muscle cells. Biochemical and Biophysical Research Communications. 2001;280(3):664–674. doi: 10.1006/bbrc.2000.4216. [DOI] [PubMed] [Google Scholar]
- 89.Parulkar AA, Pendergrass ML, Granda-Ayala R, Lee TR, Fonseca VA. Nonhypoglycemic effects of thiazolidinediones. Annals of Internal Medicine. 2001;134(1):61–71. doi: 10.7326/0003-4819-134-1-200101020-00014. [DOI] [PubMed] [Google Scholar]
- 90.Massiéra F, Bloch-Faure M, Ceiler D, et al. Adipose angiotensinogen is involved in adipose tissue growth and blood pressure regulation. The FASEB Journal. 2001;15(14):2727–2729. doi: 10.1096/fj.01-0457fje. [DOI] [PubMed] [Google Scholar]
- 91.Östgren CJ, Lindblad U, Melander O, Melander A, Groop L, Rȧstam L. Peroxisome proliferator-activated receptor-γPro12Ala polymorphism and the association with blood pressure in type 2 diabetes: skaraborg hypertension and diabetes project. Journal of Hypertension. 2003;21(9):1657–1662. doi: 10.1097/01.hjh.0000084734.53355.0d. [DOI] [PubMed] [Google Scholar]
- 92.Hasstedt SJ, Ren Q-F, Teng K, Elbein SC. Effect of the peroxisome proliferator-activated receptor-γ2 Pro12Ala variant on obesity, glucose homeostasis, and blood pressure in members of familial type 2 diabetic kindreds. Journal of Clinical Endocrinology and Metabolism. 2001;86(2):536–541. doi: 10.1210/jcem.86.2.7205. [DOI] [PubMed] [Google Scholar]
- 93.Isomaa B, Almgren P, Toumi T, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24(4):683–689. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 94.Minamikawa J, Tanaka S, Yamauchi M, Inoue D, Koshiyama H. Potent inhibitory effect of troglitazone on carotid arterial wall thickness in type 2 diabetes. Journal of Clinical Endocrinology and Metabolism. 1998;83(5):1818–1820. doi: 10.1210/jcem.83.5.4932. [DOI] [PubMed] [Google Scholar]
- 95.Tontonoz P, Nagy L, Alvarez JGA, Thomazy VA, Evans RM. PPARγ promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell. 1998;93(2):241–252. doi: 10.1016/s0092-8674(00)81575-5. [DOI] [PubMed] [Google Scholar]
- 96.Nagy L, Tontonoz P, Alvarez JGA, Chen H, Evans RM. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARγ . Cell. 1998;93(2):229–240. doi: 10.1016/s0092-8674(00)81574-3. [DOI] [PubMed] [Google Scholar]
- 97.Ricote M, Huang J, Fajas L, et al. Expression of the peroxisome proliferator-activated receptor γ (PPARγ) in human atherosclerosis and regulation in macrophages by colony stimulating factors and oxidized low density lipoprotein. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(13):7614–7619. doi: 10.1073/pnas.95.13.7614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jiang C, Ting AT, Seed B. PPAR-γ agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391(6662):82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 99.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-γ is a negative regulator of macrophage activation. Nature. 1998;391(6662):79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 100.Welch JS, Ricote M, Akiyama TE, Gonzalez FJ, Glass CK. PPARγ and PPARδ negatively regulate specific subsets of lipopolysaccharide and IFN-γ target genes in macrophages. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(11):6712–6717. doi: 10.1073/pnas.1031789100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Moore KJ, Rosen ED, Fitzgerald ML, et al. The role of PPAR-γ in macrophage differentiation and cholesterol uptake. Nature Medicine. 2001;7(1):41–47. doi: 10.1038/83328. [DOI] [PubMed] [Google Scholar]
- 102.Tall AR, Costet P, Wang N. Regulation and mechanisms of macrophage cholesterol efflux. Journal of Clinical Investigation. 2002;110(7):899–904. doi: 10.1172/JCI16391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chinetti G, Lestavel S, Bocher V, et al. PPAR-α and PPAR-γ activators induce cholesterol removal from human macrophage foam cells through stimulation of the ABCA1 pathway. Nature Medicine. 2001;7(1):53–58. doi: 10.1038/83348. [DOI] [PubMed] [Google Scholar]
- 104.Chawla A, Barak Y, Nagy L, Liao D, Tontonoz P, Evans RM. PPAR-γ dependent and independent effects on macrophage-gene expression in lipid metabolism and inflammation. Nature Medicine. 2001;7(1):48–52. doi: 10.1038/83336. [DOI] [PubMed] [Google Scholar]
- 105.Iwata E, Yamamoto I, Motomura T, et al. The association of Pro12Ala polymorphism in PPARγ2 with lower carotid artery IMT in Japanese. Diabetes Research and Clinical Practice. 2003;62(1):55–59. doi: 10.1016/s0168-8227(03)00161-x. [DOI] [PubMed] [Google Scholar]
- 106.Yan ZC, Zhu ZM, Shen CY, et al. Peroxisome proliferator-activated receptor γ C-161T polymorphism and carotid artery atherosclerosis in metabolic syndrome [in Chinese] Zhonghua Yi Xue Za Zhi. 2004;84(7):543–547. [PubMed] [Google Scholar]
- 107.Li Y, Lazar MA. Differential gene regulation by PPARγ agonist and constitutively active PPARγ2. Molecular Endocrinology. 2002;16(5):1040–1048. doi: 10.1210/mend.16.5.0825. [DOI] [PubMed] [Google Scholar]
- 108.Reginato MJ, Bailey ST, Krakow SL, et al. A potent antidiabetic thiazolidinedione with unique peroxisome proliferator-activated receptor γ-activating properties. Journal of Biological Chemistry. 1998;273(49):32679–32684. doi: 10.1074/jbc.273.49.32679. [DOI] [PubMed] [Google Scholar]
- 109.Rocchi S, Picard F, Vamecq J, et al. A unique PPARγ ligand with potent insulin-sensitizing yet weak adipogenic activity. Molecular Cell. 2001;8(4):737–747. doi: 10.1016/s1097-2765(01)00353-7. [DOI] [PubMed] [Google Scholar]
- 110.Shimaya A, Kurosaki E, Nakano R, Hirayama R, Shibasaki M, Shikama H. The novel hypoglycemic agent YM440 normalizes hyperglycemia without changing body fat weight in diabetic db/db mice. Metabolism: Clinical and Experimental. 2000;49(3):411–417. doi: 10.1016/s0026-0495(00)90440-2. [DOI] [PubMed] [Google Scholar]
- 111.Misra P, Chakrabarti R, Vikramadithyan RK, et al. PAT5A: a partial agonist of peroxisome proliferator-activated receptor γ is a potent antidiabetic thiazolidinedione yet weakly adipogenic. Journal of Pharmacology and Experimental Therapeutics. 2003;306(2):763–771. doi: 10.1124/jpet.103.049791. [DOI] [PubMed] [Google Scholar]
- 112.Berger JP, Petro AE, MacNaul KL, et al. Distinct properties and advantages of a novel peroxisome proliferator-activated protein γ selective modulator. Molecular Endocrinology. 2003;17(4):662–676. doi: 10.1210/me.2002-0217. [DOI] [PubMed] [Google Scholar]
- 113.Kim KR, Lee JH, Kim SJ, et al. KR-62980: a novel peroxisome proliferator-activated receptor γ agonist with weak adipogenic effects. Biochemical Pharmacology. 2006;72(4):446–454. doi: 10.1016/j.bcp.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 114.Liu K, Black RM, Acton JJ, III, et al. Selective PPARγ modulators with improved pharmacological profiles. Bioorganic and Medicinal Chemistry Letters. 2005;15(10):2437–2440. doi: 10.1016/j.bmcl.2005.03.092. [DOI] [PubMed] [Google Scholar]