Abstract
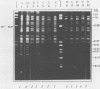
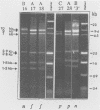
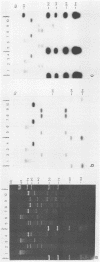
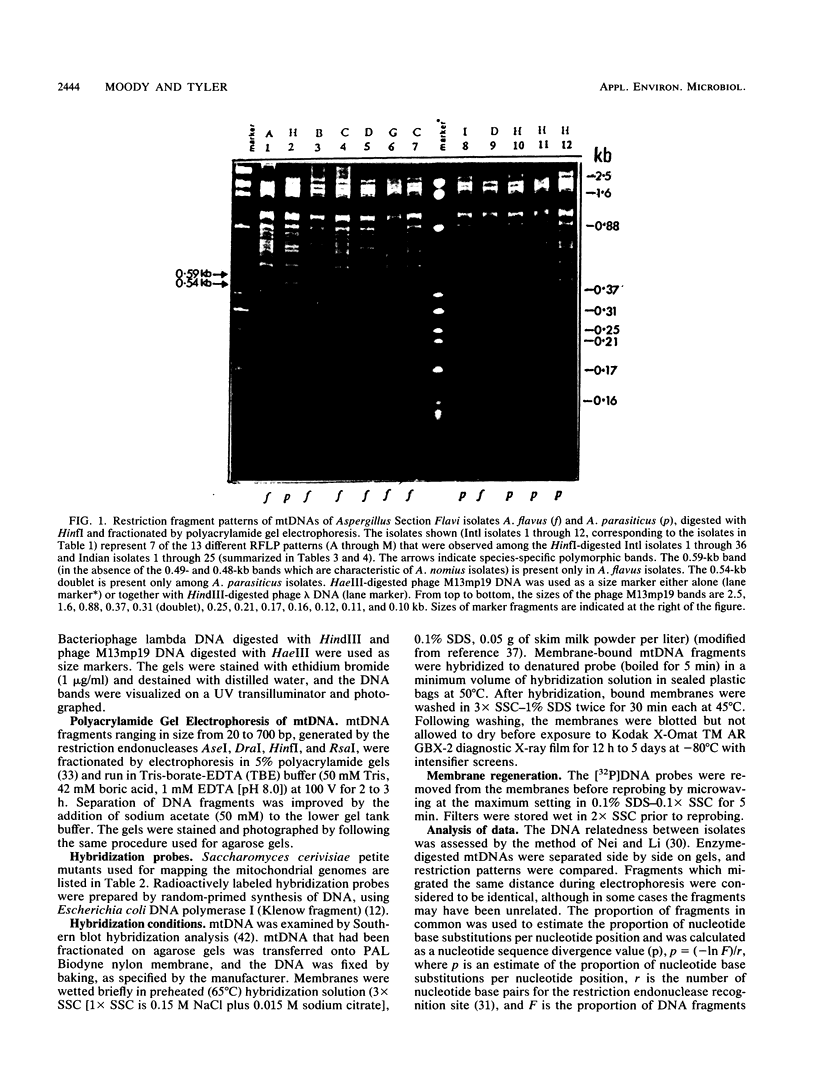
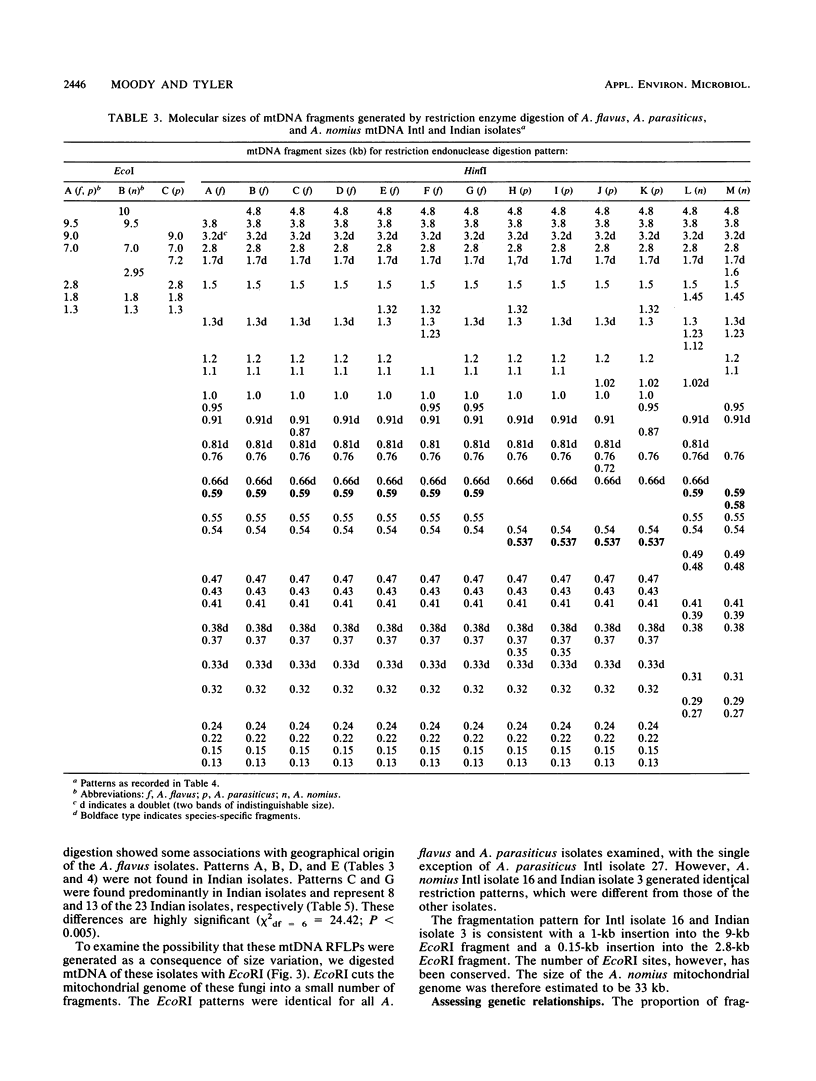
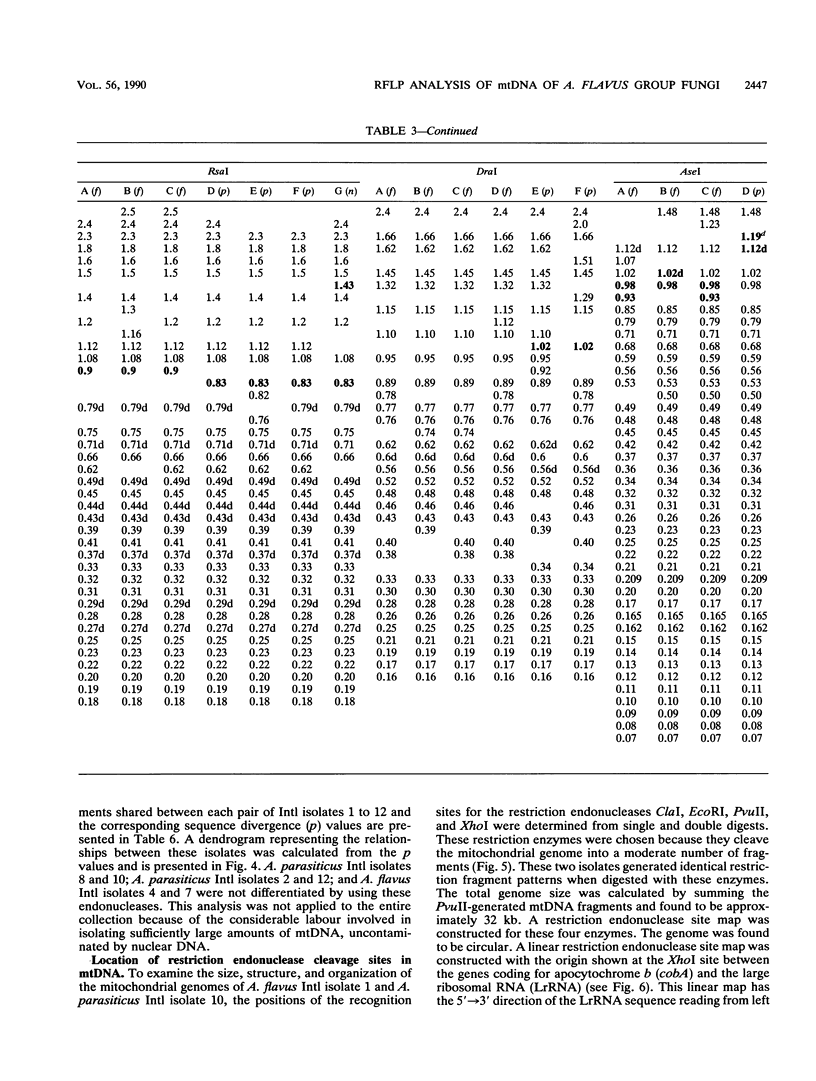
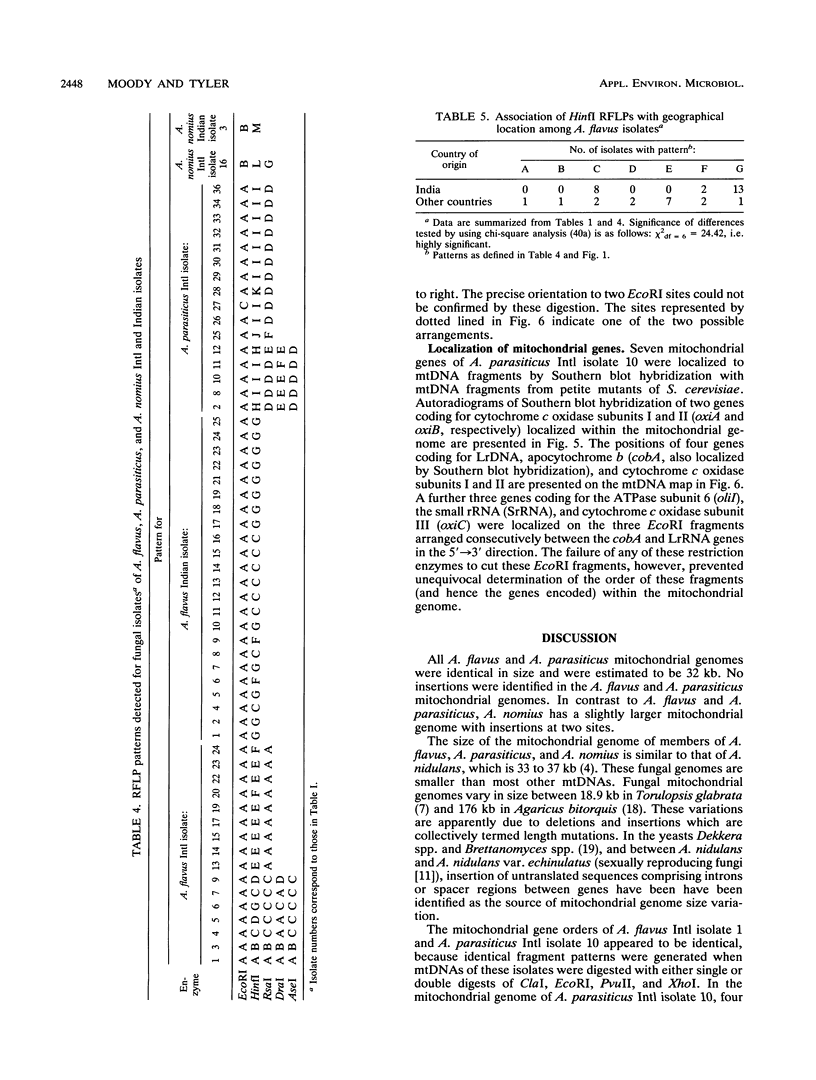
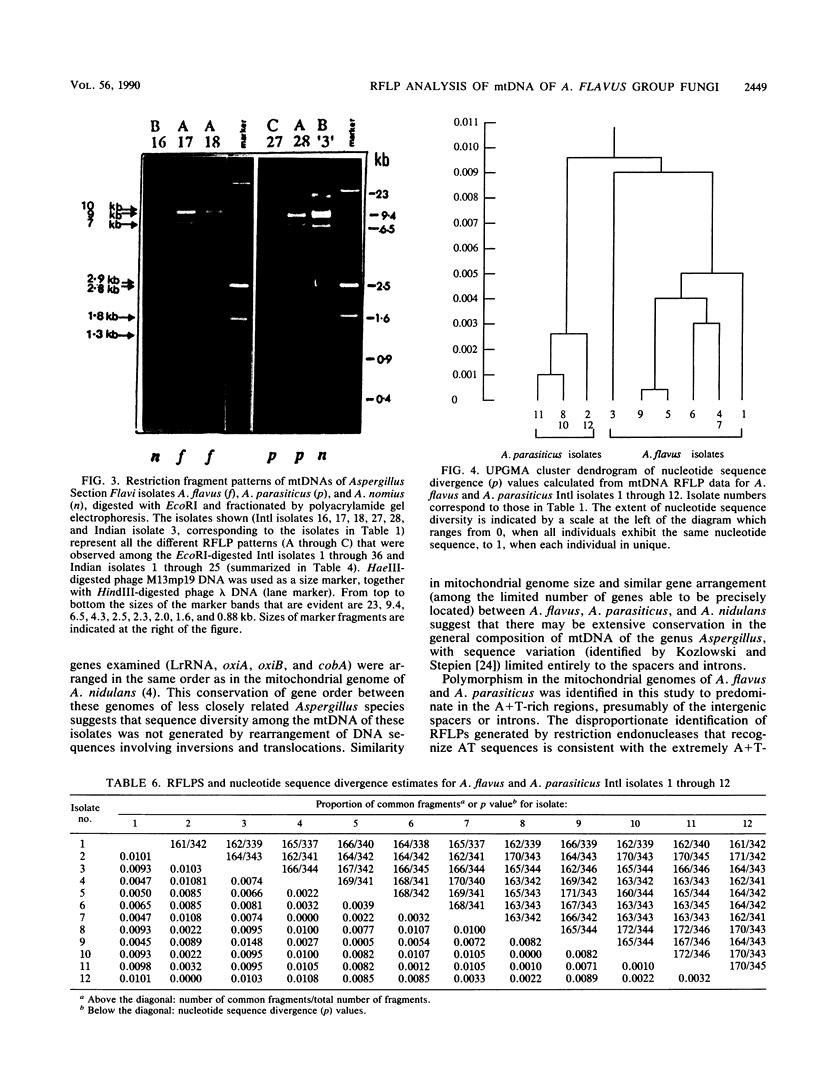
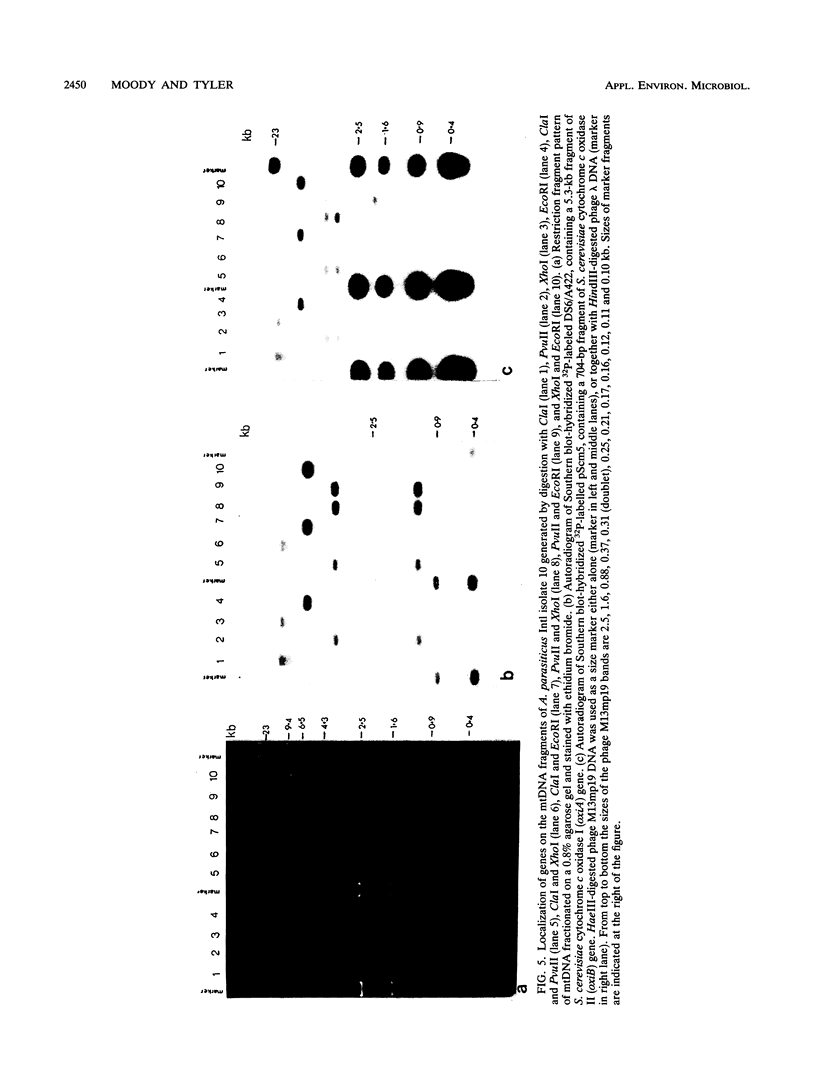
Mitochondrial DNA restriction fragment length polymorphisms were identified that clearly distinguish Aspergillus flavus, A. parasiticus, and A. nomius. Mitochondrial DNAs of A. flavus and A. parasiticus were found to be circular, and their size was estimated size to be 32 kilobases. A restriction map was constructed for the mitochondrial genome of an A. parasiticus isolate by using four restriction endonucleases. Four genes tested were found to have the same order as in the mitochondrial genome of A. nidulans. The mitochondrial genome of A. nomius was estimated to be 33 kilobases.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonitz S. G., Coruzzi G., Thalenfeld B. E., Tzagoloff A., Macino G. Assembly of the mitochondrial membrane system. Structure and nucleotide sequence of the gene coding for subunit 1 of yeast cytochrme oxidase. J Biol Chem. 1980 Dec 25;255(24):11927–11941. [PubMed] [Google Scholar]
- Brown T. A., Waring R. B., Scazzocchio C., Davies R. W. The Aspergillus nidulans mitochondrial genome. Curr Genet. 1985;9(2):113–117. doi: 10.1007/BF00436957. [DOI] [PubMed] [Google Scholar]
- Brown W. M., George M., Jr, Wilson A. C. Rapid evolution of animal mitochondrial DNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1967–1971. doi: 10.1073/pnas.76.4.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Walker G. D., McArthur C. R., Sriprakash K. S. Order and orientation of genic sequences in circular mitochondrial DNA from Saccharomyces exiguus: implications for evolution of yeast mtDNAs. J Mol Evol. 1983;19(5):333–341. doi: 10.1007/BF02101636. [DOI] [PubMed] [Google Scholar]
- Clark-Walker G. D., Sriprakash K. S. Sequence rearrangements between mitochondrial DNAs of Torulopsis glabrata and Kloeckera africana identified by hybridization with six polypeptide encoding regions from Saccharomyces cerevisiae mitochondrial DNA. J Mol Biol. 1981 Sep 25;151(3):367–387. doi: 10.1016/0022-2836(81)90002-4. [DOI] [PubMed] [Google Scholar]
- Collins R. A., Lambowitz A. M. Structural variations and optional introns in the mitochondrial DNAs of Neurospora strains isolated from nature. Plasmid. 1983 Jan;9(1):53–70. doi: 10.1016/0147-619x(83)90031-8. [DOI] [PubMed] [Google Scholar]
- Diener U. L., Davis N. D. Aflatoxin production by isolates of Aspergillus flavus. Phytopathology. 1966 Dec;56(12):1390–1393. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Garber R. C., Yoder O. C. Isolation of DNA from filamentous fungi and separation into nuclear, mitochondrial, ribosomal, and plasmid components. Anal Biochem. 1983 Dec;135(2):416–422. doi: 10.1016/0003-2697(83)90704-2. [DOI] [PubMed] [Google Scholar]
- Hoeben P., Clark-Walker G. D. An approach to yeast classification by mapping mitochondrial DNA from Dekkera/Brettanomyces and Eeniella genera. Curr Genet. 1986;10(5):371–379. doi: 10.1007/BF00418409. [DOI] [PubMed] [Google Scholar]
- Kozłowski M., Stepień P. P. Restriction enzyme analysis of mitochondrial DNA of members of the genus Aspergillus as an aid in taxonomy. J Gen Microbiol. 1982 Mar;128(3):471–476. doi: 10.1099/00221287-128-3-471. [DOI] [PubMed] [Google Scholar]
- Kurtzman C. P., Horn B. W., Hesseltine C. W. Aspergillus nomius, a new aflatoxin-producing species related to Aspergillus flavus and Aspergillus tamarii. Antonie Van Leeuwenhoek. 1987;53(3):147–158. doi: 10.1007/BF00393843. [DOI] [PubMed] [Google Scholar]
- Macino G., Tzagoloff A. Assembly of the mitochondrial membrane system: sequence analysis of a yeast mitochondrial ATPase gene containing the oli-2 and oli-4 loci. Cell. 1980 Jun;20(2):507–517. doi: 10.1016/0092-8674(80)90637-6. [DOI] [PubMed] [Google Scholar]
- Moody S. F., Tyler B. M. Use of nuclear DNA restriction fragment length polymorphisms to analyze the diversity of the Aspergillus flavus group: A. flavus, A. parasiticus, and A. nomius. Appl Environ Microbiol. 1990 Aug;56(8):2453–2461. doi: 10.1128/aem.56.8.2453-2461.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M., Li W. H. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5269–5273. doi: 10.1073/pnas.76.10.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M., Tajima F. Maximum likelihood estimation of the number of nucleotide substitutions from restriction sites data. Genetics. 1983 Sep;105(1):207–217. doi: 10.1093/genetics/105.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobrega F. G., Tzagoloff A. Assembly of the mitochondrial membrane system. DNA sequence and organization of the cytochrome b gene in Saccharomyces cerevisiae D273-10B. J Biol Chem. 1980 Oct 25;255(20):9828–9837. [PubMed] [Google Scholar]
- PONS W. A., Jr, GOLDBLATT L. A. THE DETERMINATION OF AFLATOXINS IN COTTONSEED PRODUCTS. J Am Oil Chem Soc. 1965 Jun;42:471–475. doi: 10.1007/BF02540087. [DOI] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Resolution of multiple ribonucleic acid species by polyacrylamide gel electrophoresis. Biochemistry. 1967 Jun;6(6):1818–1827. doi: 10.1021/bi00858a033. [DOI] [PubMed] [Google Scholar]
- Pitt J. I., Hocking A. D., Glenn D. R. An improved medium for the detection of Aspergillus flavus and A. parasiticus. J Appl Bacteriol. 1983 Feb;54(1):109–114. doi: 10.1111/j.1365-2672.1983.tb01307.x. [DOI] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sor F., Fukuhara H. Complete DNA sequence coding for the large ribosomal RNA of yeast mitochondria. Nucleic Acids Res. 1983 Jan 25;11(2):339–348. doi: 10.1093/nar/11.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thalenfeld B. E., Tzagoloff A. Assembly of the mitochondrial membrane system. Sequence of the oxi 2 gene of yeast mitochondrial DNA. J Biol Chem. 1980 Jul 10;255(13):6173–6180. [PubMed] [Google Scholar]