Abstract
Effective tumor immunity requires recognition of tumor cells coupled with the activation of host effector responses. Fc receptor (FcR) γ−/− mice, which lack the activating FcγR types I and III, did not demonstrate protective tumor immunity in models of passive and active immunization against a relevant tumor differentiation antigen, the brown locus protein gp75. In wild-type mice, passive immunization with mAb against gp75 or active immunization against gp75 prevented the development of lung metastases. This protective response was completely abolished in FcRγ-deficient mice. Immune responses were intact in γ−/− mice because IgG titers against gp75 develop normally in γ−/− mice immunized with gp75. However, uncoupling of the FcγR effector pathway from antibody recognition of tumor antigens resulted in a loss of protection against tumor challenge. These data demonstrate an unexpected and critical role for FcRs in mediating tumor cytotoxicity in vivo and suggest that enhancement of FcγR-mediated antibody-dependent cellular cytotoxicity by inflammatory cells is a key step in the development of effective tumor immunotherapeutics.
Effective immunity against cancer requires the specific recognition and elimination of malignant cells expressing targeted antigens. Antigens recognized on neoplastic cells include viral proteins, products of altered or mutated genes, developmentally reactivated silent gene products, and differentiation antigens expressed by tumor cells and their normal cell counterparts (1, 2). Much of the current effort of vaccine strategies is aimed at eliciting cytolytic T cell responses in which antigen recognition and cytotoxicity are functions shared by a single cell. In antibody-mediated cytotoxicity, however, antigen recognition and cytotoxicity mechanisms are functional properties of distinct cell types.
Therapeutic approaches to generate antigen-specific immune responses against tumors have included both passive immunization with mAbs and active immunization using antigens or genes expressing antigens. Passive immunity with antibodies could mediate its cytotoxic effects through complement activation or Fc receptor (FcR) engagement, and immunization with tumor antigens could elicit both cytolytic T cell responses and antibodies capable of triggering effector mechanisms. To clarify the roles of these various pathways in tumor immunity, we have examined the contributions of FcRs to the protective immune response induced against a tumor differentiation antigen by both passive and active immunization in a mouse model of tumor metastases.
Three classes of murine FcRs for IgG1, IgG2a, and IgG2b have been characterized—the high-affinity receptor FcγRI and the two low affinity receptors FcγRII and FcγRIII (3). FcγRI and III are heterooligomeric receptors, requiring coexpression of the common γ chain for their assembly and signaling functions. Cross-linking these receptors results in cell activation. FcγRII, in contrast, is a single chain inhibitory receptor, aborting activation through ITAM (immune receptor tyrosine-based activation motif) containing receptors. In addition, a distinct Fc receptor for IgG3 has been described (4, 5). Mice containing genetic disruptions of the γ chain do not express either FcγRI or III and exhibit functionally impaired antibody-mediated responses, including loss of natural killer (NK) cell-mediated antibody-dependent cellular cytotoxicity (ADCC), macrophage phagocytosis, and mast cell degranulation in response to FcR cross-linking (6). Furthermore, γ chain deficiency ameliorates the pathogenesis of cytotoxic antibody in models of autoimmune hemolytic anemia and thrombocytopenia (7, 8) and the inflammatory cascade initiated by immune complexes in the Arthus reaction (7, 9) and autoimmune glomerulonephritis (10, 11). These studies have indicated that FcRs have a dominant role in mediating the effector responses to antibodies in vivo. This study was designed to address the role of FcR-mediated effector responses in tumor immunity.
The induction of immune responses to melanoma has been associated with improved clinical outcomes in melanoma patients (12). Immunotherapeutic approaches to this disease have been actively pursued. A number of differentiation antigens have been shown to be recognized by the immune system of patients with melanoma. The melanosome, a cellular organelle found in melanoma cells and normal melanocytes, expresses several glycoproteins that are potential targets for immunity (1). In particular, the product of the brown locus (protein gp75) is expressed both intracellularly and on the cell membrane by normal melanocytes and melanoma and is recognized by T cells and autoantibodies in melanoma patients (13, 14). In a model of passive immunization against gp75 using B16F10 melanoma lung metastases, the mAb TA99 against gp75 is highly effective in preventing and eradicating early-established metastases (15). In a model of active immunization against gp75, mice immunized with recombinant mouse gp75 expressed in insect cells develop a high-titer anti-gp75 antibody response and are likewise protected in the B16F10 lung metastases model (16). In the current study, we examine the mechanism of protection in these models and conclude that the FcR-mediated effector pathway is critical in both actively and passively immunized mice for tumor rejection.
METHODS
Mice and Tumors.
γ chain-deficient mice were successively backcrossed to C57BL/6 mice (The Jackson Laboratories) for 12 generations. Six- to 8-week-old female γ chain-deficient congenic mice or wild-type (wt) C57BL/6 (The Jackson Laboratory) were used for all experiments. The B16F10 mouse melanoma cell line of C57BL/6 origin kindly provided by Isaiah Fidler (M.D. Anderson Cancer Center, Houston, TX) and were maintained in Eagle’s MEM containing 1% nonessential amino acids, penicillin (100 μg/ml), streptomycin (100 μg/ml), and 2 mM glutamine and supplemented with 5% heat-inactivated fetal bovine serum (Sigma). B16F10 melanoma cells were detached with 0.02 mM EDTA in PBS and were washed twice with PBS. Mice were injected i.v. through the tail vein with 1 × 105 B16F10 melanoma cells in 0.2 ml of sterile PBS. Mice were sacrificed 14–17 days later and lung surface metastases were counted as black nodules under a dissecting microscope.
TA99 Passive Protection Model.
Mice were injected intravenously with 105 B16 melanoma cells on day 0 and with 200 μg of purified TA99 or control mouse IgG2a mAb UPC10 (Sigma) on days 0, 2, 4, 7, 9, and 11. TA99 (IgG2a) mAb antibodies were purified from ascites fluid by protein A chromatography (Pharmacia LKB).
Sf9-gp75 Active Protection Model.
A recombinant baculovirus expression vector containing the full-length murine gp75 cDNA has been described (16). Cell suspensions of Sf9 cells (Invitrogen) infected with wt or recombinant murine gp75 baculovirus were harvested by scraping and lysates prepared by three successive freeze–thaw cycles. Initial intraperitoneal immunizations were in complete Freund’s adjuvant and the subsequent three intraperitoneal immunizations at 2-week intervals were in incomplete Freund’s adjuvant (Sigma). Four weeks after the last immunization serum was obtained to check antibody responses and the mice were injected intravenously through the tail vein with 105 B16 melanoma cells.
Immunoprecipitation.
Precleared serum obtained from immunized mice were mixed with [35S]methionine-labeled B16F10 lysates (3–10 × 106 cpm of trichloroacetic acid-insoluble precipitate) and pelleted with protein A-Sepharose. Disrupted complexes were subjected to denaturing PAGE and autoradiography. Purified anti-gp75 mAb TA99 was used as a positive control.
Macrophage-Mediated ADCC.
Peritoneal macrophages were obtained from mice immunized with live attenuated bacillus Calmette–Guérin (Organon Teknika–Cappel) subcutaneously in complete Freund’s adjuvant followed by i.p. administration 6 weeks later. Approximately 107 macrophages were obtained per animal 7 days after i.p. inoculation. Macrophages were cultured at an effector/target ratio of 10:1 (105 effector to 104 target cells per well) in 96-well plates. Target cells were chromium-51-labeled HSB-2 lymphoma cells derivitized with 2,4,6-trinitrophenyl (TNP) and opsonized with subagglutinating quantities of anti-TNP hybridoma supernatants. TNP-specific hybridomas included TIB191 (IgG1) obtained from the American Type Culture Collection and U7.12 (IgG2a) and U12.5 (IgG2b) both obtained from Jay Unkeless (Mt. Sinai Medical Center, New York, NY). ADCC reactions were for 6 h and specific activities were obtained as [(cpm from cultures with antibody) − (cpm from cultures with medium)]/(total cpm). Samples were assayed in triplicate with results expressed as the mean ± SEM.
RESULTS
FcR Is Required for Passive Protection of Melanoma Metastases by mAb TA99.
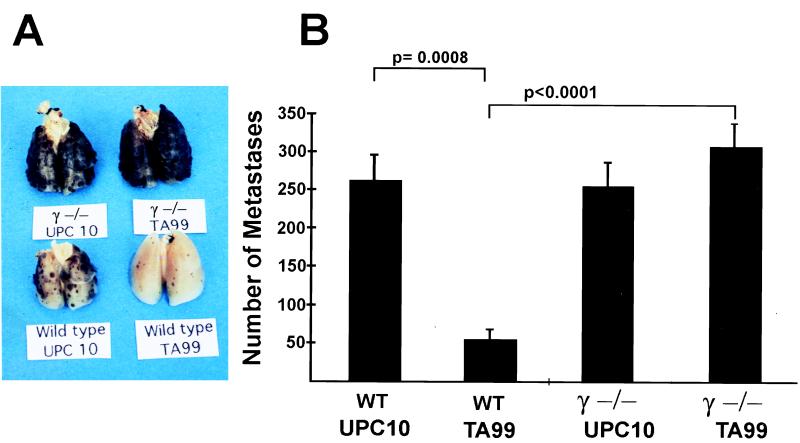
To determine the in vivo consequences of the loss of FcγRs in tumor immunity, γ−/− C57BL/6 congenic mice were developed by 12 successive backcrosses to the FcRγ−/− mixed background (129/Bl.6). To show that the genetic background of the congenic line was phenotypically similar to C57BL/6 mice, C57BL/6 γ+/− heterozygous mice were compared with wt C57BL/6 mice for baseline susceptibility to lung metastases in the B16F10 melanoma model. The number of lung metastases in congenic γ+/− mice were found to be similar to wt C57BL/6 mice (195 ± 15 vs. 187 ± 20 nodules). In addition, both strains were similarly protected from lung metastases by passive immunization with mAb TA99 against gp75 (85% vs. 78% reduction). Deletion of FcγRI and -III by disruption of the common γ chain, however, results in loss of the protective effect of TA99, as shown in Fig. 1, indicating that FcγR effector pathways are necessary for tumor rejection in mice passively immunized with mAb TA99.
Figure 1.
Passive protection from melanoma metastases requires FcRs. (A) Representative lungs from wt and γ−/− mice injected i.v. with 105 B16 melanoma cells. Mice were injected with either anti-gp75 (TA99) or control isotype antibody (UPC10). Six mice were present in each group. (B) Data are the mean ± SEM. P values of significant differences (Fisher exact test) are noted.
FcR Is Required for Protection of Melanoma Metastases by Active Vaccination.
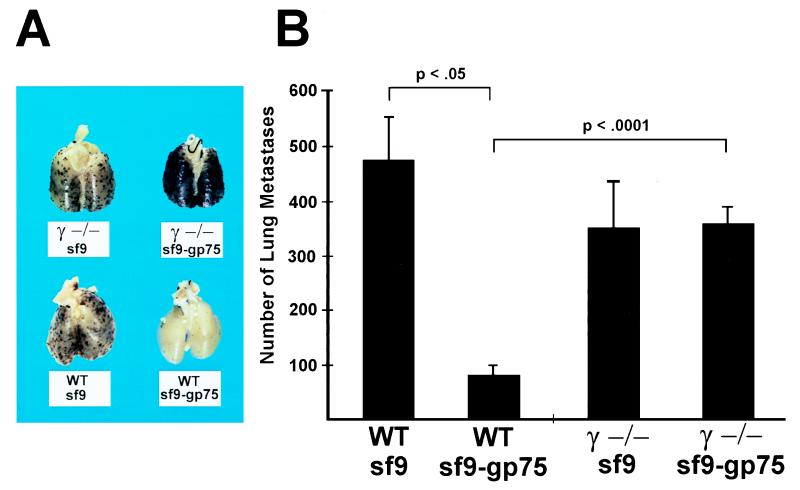
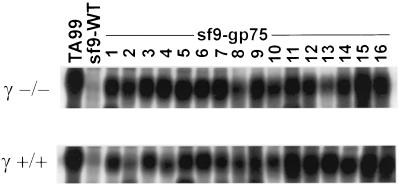
In comparison with passive immunization, the situation in actively immunized mice is far more complex and is expected to include the polyclonal induction of both anti-gp75 T cells and antibodies, thus providing the host a number of possible cytotoxic effector systems including both cytotoxic T lymphocyte-mediated and antibody-mediated pathways. To determine the importance of the antibody-mediated FcγR effector pathway in actively immunized mice, γ−/− and wild-type (wt) mice were immunized with syngeneic gp75 expressed in cellular extracts of insect cells infected with mouse gp75 baculovirus constructs or with wt Sf9 cellular extracts. One consequence of Sf9-gp75 immunization is the induction of autoimmune depigmentation. This coat-color change recapitulates a possible clinical association of prolonged survival and vitiligo in melanoma patients (17–21). The typical appearance of depigmentation occurred in both wt and γ−/− Sf9-gp75-immunized mice, indicating that the effector arm of the anti-melanocyte immune response, probably T cell-mediated (15), does not require an intact FcγR γ chain (Fig. 2). Distinct from this autoimmune phenomenon, the anti-tumor effects of Sf9-gp75 immunization were strikingly different in the two strains of mice. Consistent with prior studies, Sf9-gp75-immunized wt mice had a significant reduction in lung metastases, with 83% fewer nodules (Fig. 3). In contrast Sf9-gp75-immunized γ−/− mice were afforded no protection against melanoma metastases. γ−/− mice have been shown to have normal immune responses to a variety of antigenic challenges (6, 22). This same situation is seen in the γ−/− congenic mice used in these studies because easily detectable anti-gp75 IgG were found in Sf9-gp75-immunized wt and γ−/− mice (Fig. 4). Immunized γ−/− congenic mice exhibited normal CD4 and CD8 T cell responses when immunized with PCC (pigeon cytochrome c) peptide and OVA (ovalbumin) peptide, respectively. Class II-restricted T cell proliferative responses of CD4 + LN cells from wt and γ−/−-immunized mice were comparable when cocultured with PCC peptide (data not shown). Both wt and γ-deficient splenocytes were capable of T cell-mediated OVA-specific killing of OVA-pulsed EL-4 target cells at comparable effector/target ratios, thereby demonstrating that the cytolytic CD8+ T cell response is unaltered in γ-deficient mice (data not shown). Thus, these experiments demonstrate that both T cell immune recognition (anti-PCC response) and effector responses (anti-OVA-pulsed EL-4 cytotoxicity) are intact in γ−/− mice. Despite the fact that these mice are capable of developing normal B and T cell immune responses, the genetic disruption of the γ-mediated effector pathway is sufficient to abrogate the efficacy of a tumor vaccine. In the absence of FcγR, a requisite receptor for antibody-mediated tumor cytotoxicity, anti-melanoma responses are rendered incapable of tumor protection.
Figure 2.
Anti-gp75-induced depigmentation occurs normally in FcγR-deficient mice. γ−/− and wt mice were immunized with Sf9-gp75 (gray mice) or with control Sf9 extract (black mice).
Figure 3.
Active protection from melanoma metastases requires FcRs. (A) Representative lungs from wt and γ−/− mice injected i.v. with 105 B16 melanoma cells. Mice were immunized with either anti-gp75 (Sf9-gp75) or control extract (Sf9). Six mice were present in each group. (B) Data are the mean ± SEM. P values of significant differences (Fisher exact test) are noted.
Figure 4.
Anti-gp75 titers in Sf9-gp75-immunized γ+/+ and γ−/− mice are indistinguishable. Immunoprecipitations of diluted serum samples from Sf9-immunized (negative control) and Sf9-gp75-immunized mice. Diluted TA99 anti-gp75 mAb is used as a positive control.
Macrophage-Mediated ADCC Is Abolished in γ−/− Mice.
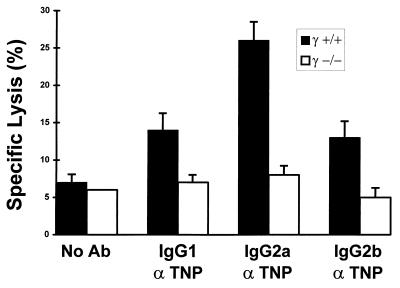
To determine the mechanism by which FcR-deficient mice are unable to mediate an ADCC response, FcR-expressing effector cells were studied in vitro. Both NK cells and myeloid cells express FcRs and are capable of antibody-mediated tumor cytotoxicity. In vitro data has indicated that ADCC mediated by NK cells, which express only the type III FcγR, is abolished in γ-chain deficient mice (6). It is unlikely that only NK cells are involved in ADCC in the B16 murine melanoma model, because studies have shown (23) that SCID/Beige mice, which lack NK cytolytic capacity, are capable of sustaining a TA99-mediated protective response. To ascertain whether macrophages, which express all three FcγRs, also require the γ chain for anti-tumor ADCC, bacillus Calmette–Guérin-activated peritoneal macrophages were cocultured with TNP-derivitized HSB-2 tumor target cells in the presence of anti-TNP antibodies. Unlike the situation with TA99 and B16F10 tumor target cells (16, 23), this system efficiently produces ADCC reactions. Whereas wt macrophages killed 27% of IgG2a-opsonized HSB-2 tumor cells, there was no enhancement of γ−/− macrophage-mediated cytotoxicity with IgG1, IgG2a, or IgG2b opsonization (Fig. 5). Therefore, both NK and monocyte lineage effectors require the γ chain for affective ADCC of tumor target cells in vitro, suggesting that both cellular populations may be significant in tumor immunity and thus compromised in γ−/− mice.
Figure 5.
Macrophage ADCC of tumor target cells requires FcγR γ chain. Bacillus Calmette–Guérin-elicited peritoneal γ+/+ and γ−/− macrophages were cultured with chromium-labeled TNP-opsonized HSB-2 target cells at an effector/target ratio of 10:1. Data are expressed as the mean ± SEM of triplicate samples assayed in the presence or absence of subagglutinating quantities of IgG1, IgG2a, or IgG2b anti-dinitrophenyl antibody.
DISCUSSION
The challenge of cancer immunotherapy is the induction of anti-tumor immune responses specific for self or altered-self tumor antigens. The resultant immune responses are, therefore, capable of triggering both clinical tumor responses and autoimmune phenomena. In this study the effector mechanisms responsible for these outcomes are explored in a melanoma model in which immunization can induce both anti-tumor responses and autoimmune vitiligo. The cytotoxic response mediated by antibodies is just one possible effector mechanism contributing to the efficacy of anti-tumor mAbs or tumor vaccines. Indeed, much of the current effort in tumor immunology is directed at the generation of effective cytolytic T cell responses. In the protection model described herein, however, the critical requirement for FcγR suggests that the development of cytotoxic IgG is the dominant anti-melanoma mechanism. Although it remains to be demonstrated that this is the case with other tumor vaccines, the results of this study presented herein imply that the FcγR-deficient mouse is a novel assay system that evaluates the role of cytotoxic IgG in immunotherapeutics.
The persistance of depigmentation induction in γ−/− mice reveals a distinction between the anti-tumor and anti-melanocyte effector pathways. Although antibody-mediated responses have been implicated in the pathogenesis of vitiligo (19, 20, 24–28), the data presented herein are more consistent with prior Thy1.2 depletion studies (15) in which depigmentation was abrogated in gp75-immunized mice and suggest instead a role for T cell responses rather than cytotoxic IgG in the anti-melanocyte autoimmune response. The dissociation of depigmentation from tumor immunity in γ−/− mice argues that the anti-melanocyte response is not sufficient to convey tumor immunity and suggests that the clinical correlation of vitiligo with tumor responses is not necessarily the result of a shared immunological response.
The identity of the FcR-bearing effector cell that mediates the anti-gp75 cytotoxicity in tumor protection is currently unknown. Prior cell depletion experiments showed that both NK1.1-bearing cells and to a much lesser extent, CD4+ cells were required for TA99 protection (15). On the other hand in the SCID/Beige mouse, which lacks mature T and B cells, as well as NK cells with cytolytic capacity, tumor protection by mAb TA99 is intact (23), suggesting instead that cytotoxic T lymphocyte- and NK-mediated cytotoxicity are not required. These findings can be reconciled with the hypotheses that macrophage-mediated ADCC is critical to anti-tumor efficacy and that CD4+ and NK1.1+ cells are required as immunoregulatory cells for stimulation of macrophage activity. The lack of ADCC by γ−/− macrophages is consistent with this hypothesis.
Regardless of the lineage of the effector cell(s) involved, these studies substantiate a critical role in vivo for FcγR-mediated ADCC in tumor immunity. Although anti-gp75-mediated ADCC has not been demonstrated in vitro, data have shown that transfer of serum from Sf9-gp75-immunized mice can protect naïve mice from melanoma metastases, suggesting that serum anti-gp75 IgG is sufficient to provide protection (16, 23). The evidence presented herein indicates that the FcγR effector pathway is the dominant mechanism of protection of this humoral response and is necessary for the efficacy of a tumor vaccine. Uncoupling of the FcγR pathway from antibody recognition of tumor antigens resulted in a loss of protection against tumor challenge. These observations suggest that enhancement of the functional activity of anti-tumor antibody–FcγR interactions would improve the efficacy of immunotherapeutic agents.
Acknowledgments
We thank the members of the Ravetch and Houghton labs for helpful discussions, and Fred Vital and Cynthia Ritter for technical and administrative assistance. This work was supported by Swim Across America (A.H.), Louis and Anne Abrons (A.H.), and National Institutes of Health Grants K08DK02468 (R.C.), R01CA56821 (A.H.), and R01AI35875 (J.V.R.).
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: FcR, Fc receptor; NK, natural killer; ADCC, antibody-dependent cellular cytotoxicity; TNP, trinitrophenyl; wt, wild type.
References
- 1.Houghton A N. J Exp Med. 1994;180:1–4. doi: 10.1084/jem.180.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaffee E M, Perdoll D M. Curr Opin Immunol. 1996;8(5):622–627. doi: 10.1016/s0952-7915(96)80077-x. [DOI] [PubMed] [Google Scholar]
- 3.Ravetch J V. Curr Opin Immunol. 1997;9:121–125. doi: 10.1016/s0952-7915(97)80168-9. [DOI] [PubMed] [Google Scholar]
- 4.Diamond B, Yelton D E. J Exp Med. 1981;153:514–519. doi: 10.1084/jem.153.3.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuan, R., Clynes, R., Oh, J., Ravetch, J. V. & Scharff, M. D. (1998) J. Exp. Med., in press. [DOI] [PMC free article] [PubMed]
- 6.Takai T, Li M, Sylvestre D, Clynes R, Ravetch J V. Cell. 1994;76:519–529. doi: 10.1016/0092-8674(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 7.Clynes R, Sylvestre D, Ma M, Warren H, Carroll M C, Ravetch J V. J Exp Med. 1996;184:2385–2392. doi: 10.1084/jem.184.6.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clynes R, Ravetch J V. Immunity. 1995;3:21–26. doi: 10.1016/1074-7613(95)90155-8. [DOI] [PubMed] [Google Scholar]
- 9.Sylvestre D L, Ravetch J V. Science. 1994;265:1095–1098. doi: 10.1126/science.8066448. [DOI] [PubMed] [Google Scholar]
- 10.Clynes, R., Dumitru, C. & Ravetch, J. V. (1998) Science, in press. [DOI] [PubMed]
- 11.Suzuki, Y., Shirato, I., Tomin, Y., Okomura, K., Ravetch, J. V., Takai, T. & Ra, C. (1998) J. Clin. Invest., in press. [DOI] [PubMed]
- 12.Livingston P O, Wong G Y, Adluri S, Tao Y, Padavan M, Parente R, Hanlon C, Calves M J, Helling F, Ritter G, Oettgen H F, Old L J. J Clin Oncol. 1994;12:1036–1044. doi: 10.1200/JCO.1994.12.5.1036. [DOI] [PubMed] [Google Scholar]
- 13.Wang R F, Robbins P F, Kawakami Y, Kang X Q, Rosenberg S A. J Exp Med. 1995;181:799–804. doi: 10.1084/jem.181.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vijayasaradhi S, Bouchard B, Houghton A N. J Exp Med. 1990;171:1375–1380. doi: 10.1084/jem.171.4.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hara I, Takechi Y, Houghton A N. J Exp Med. 1995;182:1609–1614. doi: 10.1084/jem.182.5.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naftzger C, Takechi Y, Kohda H, Hara I, Vijayasaradhi S, Houghton A N. Proc Natl Acad Sci USA. 1996;93:14809–14814. doi: 10.1073/pnas.93.25.14809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nordlund J J, Kirkwood J M, Forget B M, Milton G, Albert D M, Lerner A B. J Am Acad Dermatol. 1983;9:689–696. doi: 10.1016/s0190-9622(83)70182-9. [DOI] [PubMed] [Google Scholar]
- 18.Duhra P, Ilchyshyn A. Clin Exp Dermatol. 1991;16:303–305. doi: 10.1111/j.1365-2230.1991.tb00383.x. [DOI] [PubMed] [Google Scholar]
- 19.Cui J, Bystryn J C. Arch Dermatol. 1995;131:314–318. [PubMed] [Google Scholar]
- 20.Merimsky O, Shoenfeld Y, Baharav E, Altomonte M, Chaitchik S, Maio M, Ferrone S, Fishman P. Am J Clin Oncol. 1996;19:613–618. doi: 10.1097/00000421-199612000-00017. [DOI] [PubMed] [Google Scholar]
- 21.Rosenberg S A, White D E. J Immunother Emphasis Tumor Immunol. 1996;19:81–84. [PubMed] [Google Scholar]
- 22.Vora K A, Ravetch J V, Manser T. J Immunol. 1997;159:2116–2124. [PubMed] [Google Scholar]
- 23.Takechi Y, Hara I, Naftzger C, Xu Y Q, Houghton A N. Clin Cancer Res. 1996;2:1837–1842. [PubMed] [Google Scholar]
- 24.Fishman P, Merimsky O, Baharav E, Shoenfeld Y. Cancer. 1997;79:1461–1464. doi: 10.1002/(sici)1097-0142(19970415)79:8<1461::aid-cncr3>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 25.Hann S K, Koo S W, Kim J B, Park Y K. J Dermatol. 1996;23:100–103. [PubMed] [Google Scholar]
- 26.Merimsky O, Baharav E, Shoenfeld Y, Chaitchik S, Tsigelman R, Cohen-Aloro D, Fishman P. Cancer Immunol Immunother. 1996;42:297–302. doi: 10.1007/s002620050286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hann S K, Kim J B. Yonsei Med J. 1995;36:457–461. doi: 10.3349/ymj.1995.36.5.457. [DOI] [PubMed] [Google Scholar]
- 28.Merimsky O, Shoenfeld Y, Yecheskel G, Chaitchik S, Azizi E, Fishman P. Cancer Immunol Immunother. 1994;38:411–416. doi: 10.1007/BF01517212. [DOI] [PMC free article] [PubMed] [Google Scholar]