Abstract
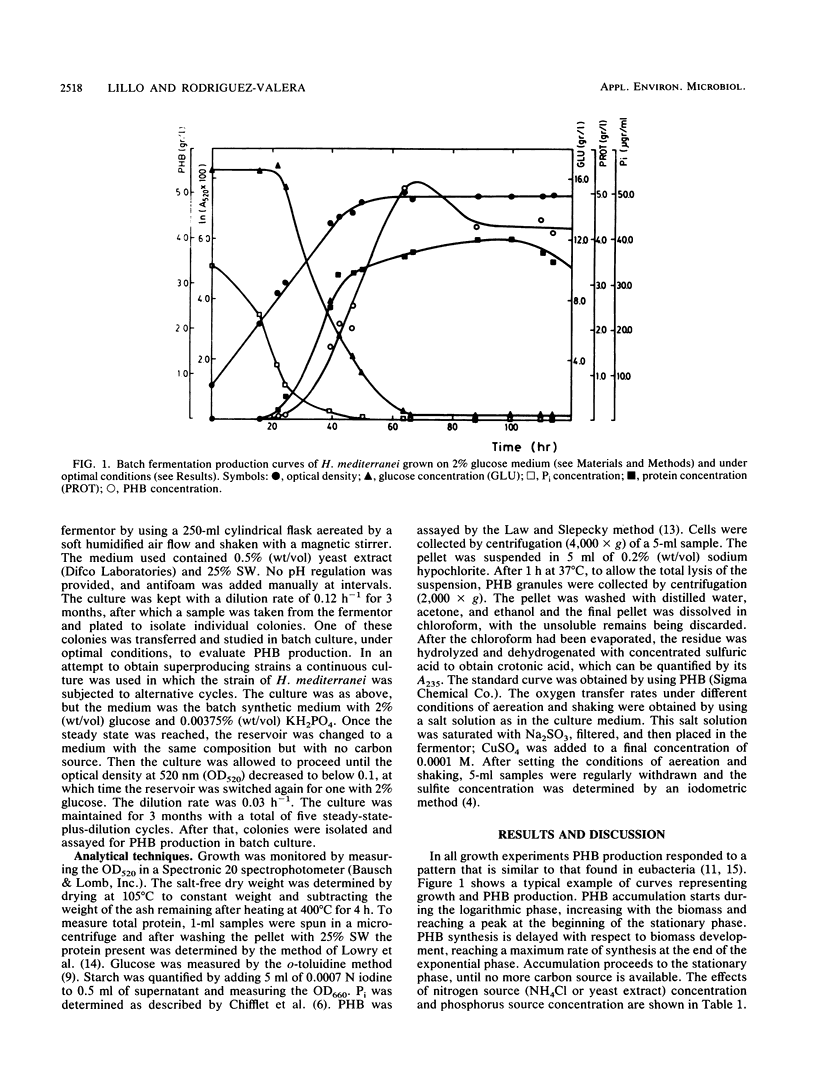
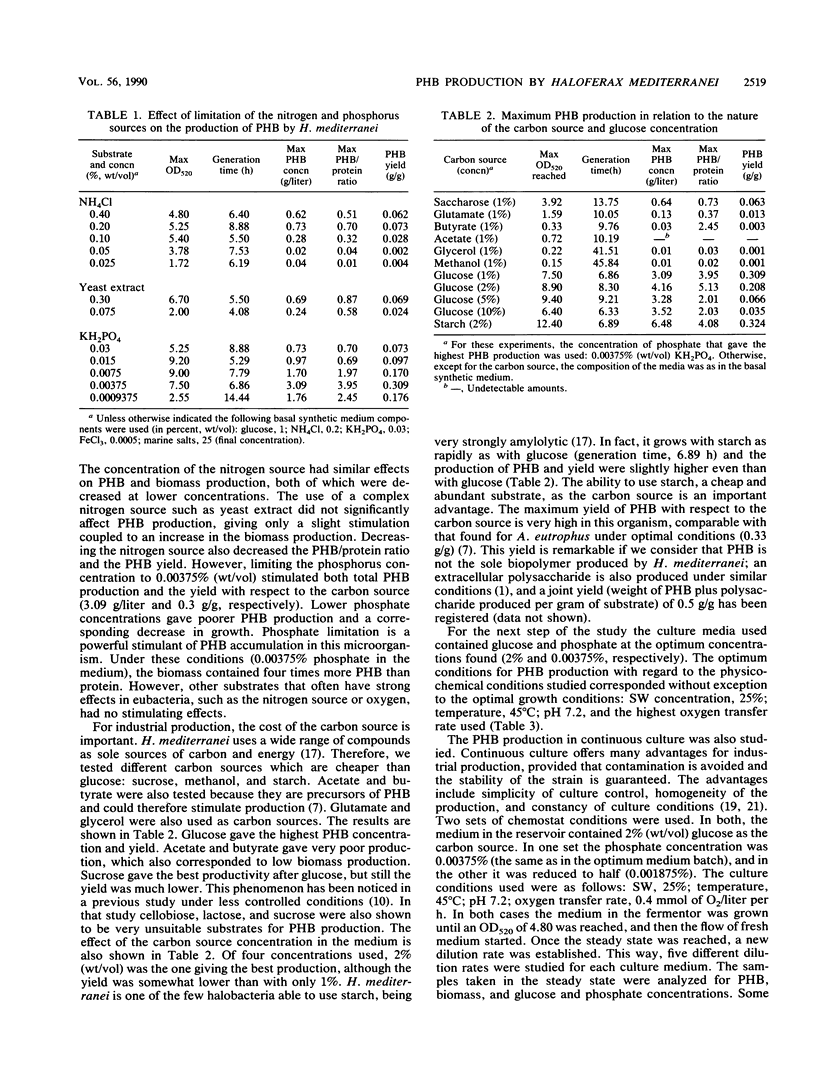
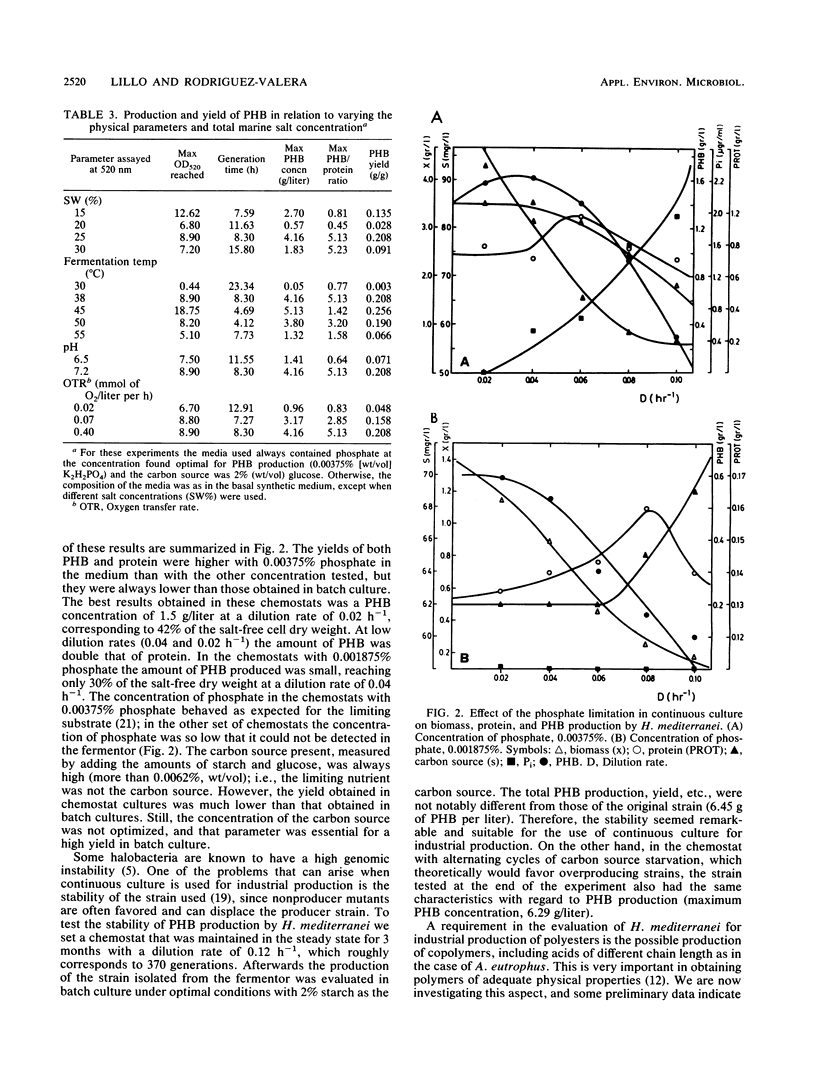
The halobacterium Haloferax mediterranei accumulates poly(β-hydroxybutyrate) (PHB) as intracellular granules. The conditions for PHB production in batch and continuous cultures have been studied and optimized. Phosphate limitation is essential for PHB accumulation in large quantities. Glucose and starch are the best carbon sources. With 2% starch, 0.00375% KH2PO4, and 0.2% NH4Cl in batch culture, a production of ca. 6 g of PHB per liter was reached, being 60% of the total biomass dry weight, and giving a yield over the carbon source of 0.33 g/g. The PHB production in continuous cultures was stable over a 3-month period. Our results demonstrate that H. mediterranei is an interesting candidate for industrial production of biological polyesters.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antón J., Meseguer I., Rodríguez-Valera F. Production of an Extracellular Polysaccharide by Haloferax mediterranei. Appl Environ Microbiol. 1988 Oct;54(10):2381–2386. doi: 10.1128/aem.54.10.2381-2386.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandl H., Gross R. A., Lenz R. W., Fuller R. C. Pseudomonas oleovorans as a Source of Poly(beta-Hydroxyalkanoates) for Potential Applications as Biodegradable Polyesters. Appl Environ Microbiol. 1988 Aug;54(8):1977–1982. doi: 10.1128/aem.54.8.1977-1982.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chifflet S., Torriglia A., Chiesa R., Tolosa S. A method for the determination of inorganic phosphate in the presence of labile organic phosphate and high concentrations of protein: application to lens ATPases. Anal Biochem. 1988 Jan;168(1):1–4. doi: 10.1016/0003-2697(88)90002-4. [DOI] [PubMed] [Google Scholar]
- DUBOWSKI K. M. An o-toluidine method for body-fluid glucose determination. Clin Chem. 1962 May-Jun;8:215–235. [PubMed] [Google Scholar]
- Dawes E. A., Senior P. J. The role and regulation of energy reserve polymers in micro-organisms. Adv Microb Physiol. 1973;10:135–266. doi: 10.1016/s0065-2911(08)60088-0. [DOI] [PubMed] [Google Scholar]
- Fernandez-Castillo R., Rodriguez-Valera F., Gonzalez-Ramos J., Ruiz-Berraquero F. Accumulation of Poly (beta-Hydroxybutyrate) by Halobacteria. Appl Environ Microbiol. 1986 Jan;51(1):214–216. doi: 10.1128/aem.51.1.214-216.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAW J. H., SLEPECKY R. A. Assay of poly-beta-hydroxybutyric acid. J Bacteriol. 1961 Jul;82:33–36. doi: 10.1128/jb.82.1.33-36.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ramsay B. A., Ramsay J. A., Cooper D. G. Production of Poly-beta-Hydroxyalkanoic Acid by Pseudomonas cepacia. Appl Environ Microbiol. 1989 Mar;55(3):584–589. doi: 10.1128/aem.55.3.584-589.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]


