Abstract
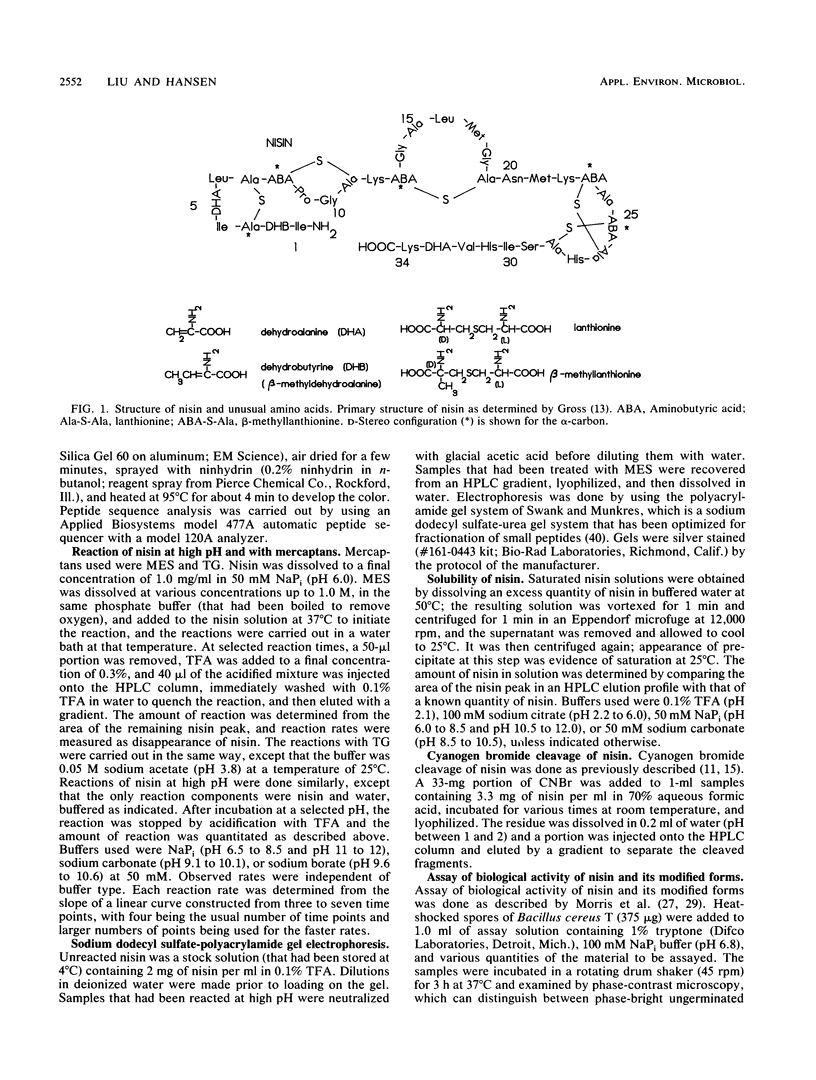
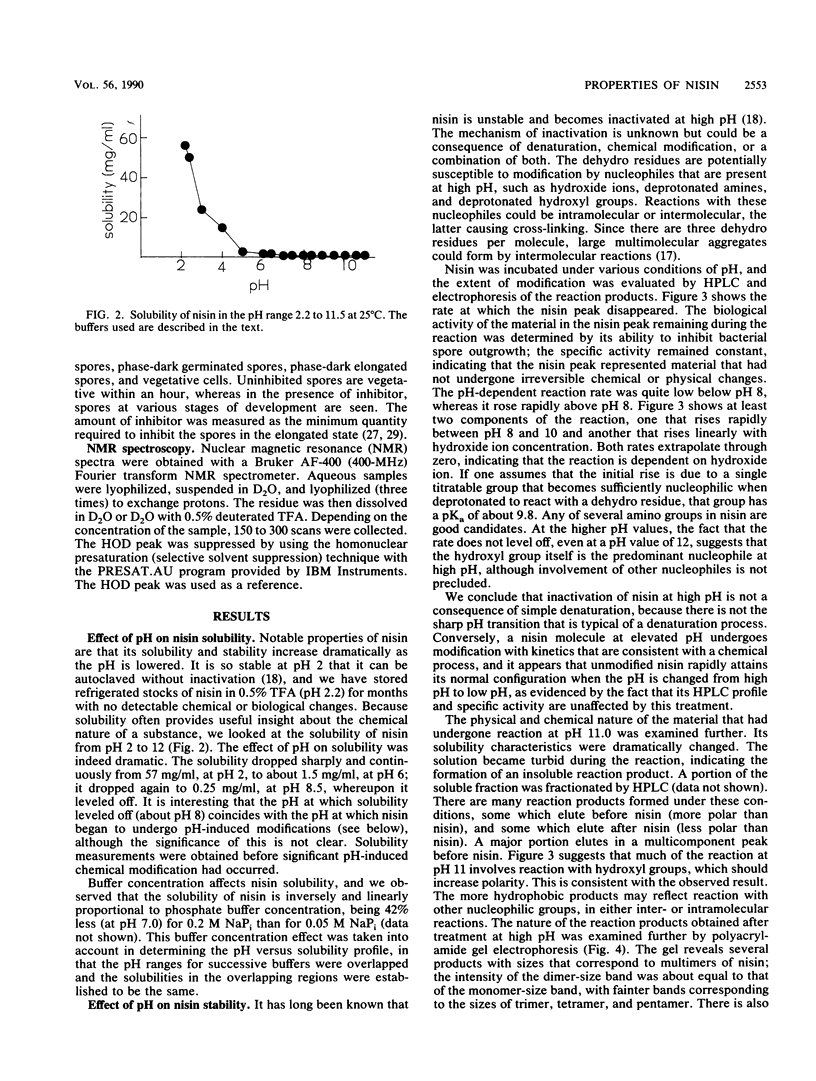
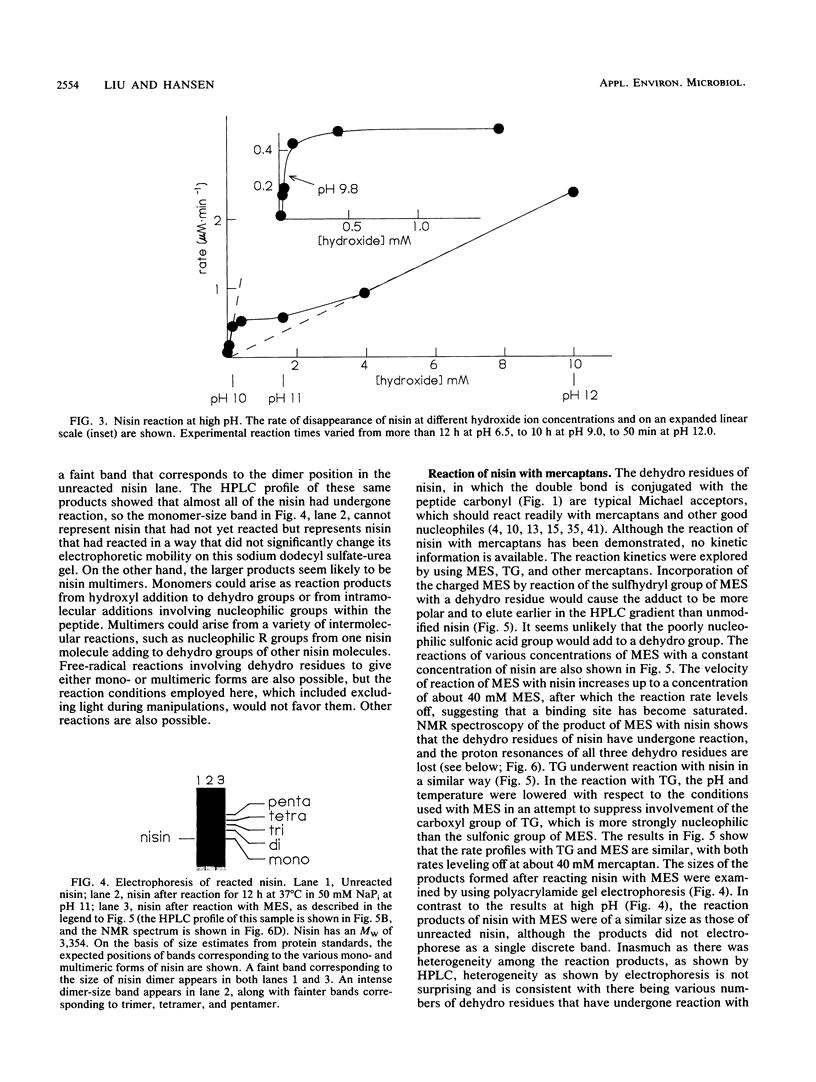
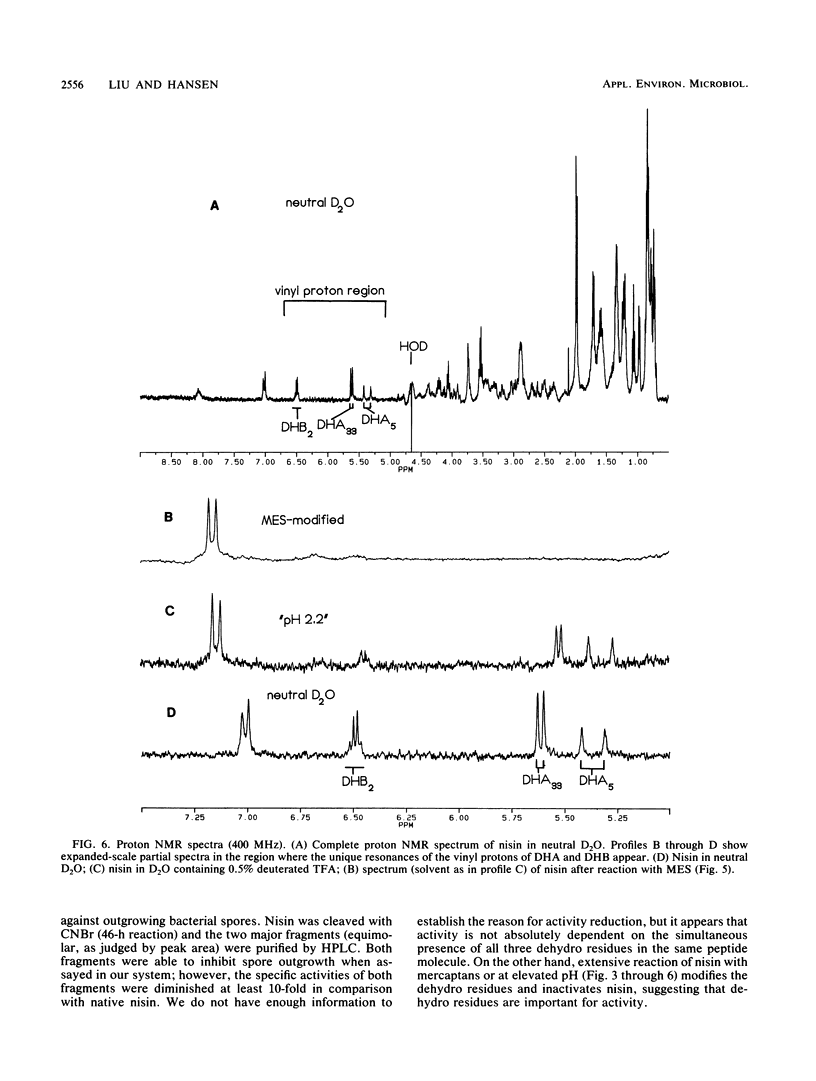
Nisin is a small gene-encoded antimicrobial protein produced by Lactococcus lactis that contains unusual dehydroalanine and dehydrobutyrine residues. The reactivity of these residues toward nucleophiles was explored by reacting nisin with a variety of mercaptans. The kinetics of reaction with 2-mercaptoethane-sulfonate and thioglycolate indicated that the reaction pathway includes a binding step. Reaction of nisin at high pH resulted in the formation of multimeric products, apparently as a result of intramolecular and intermolecular reactions between nucleophilic groups and the dehydro residues. One of the nucleophiles had a pKa of about 9.8. The unique vinyl protons of the dehydro residues that give readily identifiable proton nuclear magnetic resonances were used to observe the addition of nucleophiles to the dehydro moiety. After reaction with nucleophiles, nisin lost its antibiotic activity and no longer showed the dehydro resonances, indicating that the dehydro groups had been modified. The effect of pH on the solubility of nisin was determined; the solubility was quite high at low pH (57 mg/ml at pH 2) and was much lower at high pH (0.25 mg/ml at pH 8 to 12), as measured before significant pH-induced chemical modification had occurred. High-performance liquid chromatography on a C18 column was an effective technique for separating unmodified nisin from its reaction products. The cyanogen bromide cleavage products of nisin were about 90% less active toward inhibition of bacterial spore outgrowth than was native nisin. These results are consistent with earlier observations, which suggested that the dehydro residues of nisin have a role in the mechanism of antibiotic action, in which they act as electrophilic Michael acceptors toward nucleophiles in the cellular target.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allgaier H., Jung G., Werner R. G., Schneider U., Zähner H. Epidermin: sequencing of a heterodetic tetracyclic 21-peptide amide antibiotic. Eur J Biochem. 1986 Oct 1;160(1):9–22. doi: 10.1111/j.1432-1033.1986.tb09933.x. [DOI] [PubMed] [Google Scholar]
- Banerjee S., Hansen J. N. Structure and expression of a gene encoding the precursor of subtilin, a small protein antibiotic. J Biol Chem. 1988 Jul 5;263(19):9508–9514. [PubMed] [Google Scholar]
- Buchman G. W., 3rd, Hansen J. N. Modification of membrane sulfhydryl groups in bacteriostatic action of nitrite. Appl Environ Microbiol. 1987 Jan;53(1):79–82. doi: 10.1128/aem.53.1.79-82.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman G. W., Banerjee S., Hansen J. N. Structure, expression, and evolution of a gene encoding the precursor of nisin, a small protein antibiotic. J Biol Chem. 1988 Nov 5;263(31):16260–16266. [PubMed] [Google Scholar]
- Gross E., Morell J. L. The number and nature of alpha,beta-unsaturated amino acids in nisin. FEBS Lett. 1968 Nov;2(1):61–64. doi: 10.1016/0014-5793(68)80101-2. [DOI] [PubMed] [Google Scholar]
- Gross E., Morell J. L. The presence of dehydroalanine in the antibiotic nisin and its relationship to activity. J Am Chem Soc. 1967 May 24;89(11):2791–2792. doi: 10.1021/ja00987a084. [DOI] [PubMed] [Google Scholar]
- Gross E., Morell J. L. The structure of nisin. J Am Chem Soc. 1971 Sep 8;93(18):4634–4635. doi: 10.1021/ja00747a073. [DOI] [PubMed] [Google Scholar]
- Gross E. alpha, beta-Unsaturated and related amino acids in peptides and proteins. Adv Exp Med Biol. 1977;86B:131–153. doi: 10.1007/978-1-4757-9113-6_9. [DOI] [PubMed] [Google Scholar]
- Jones A. J., Helmerhorst E., Stokes G. B. The formation of dehydroalanine residues in alkali-treated insulin and oxidized glutathione. A nuclear-magnetic-resonance study. Biochem J. 1983 May 1;211(2):499–502. doi: 10.1042/bj2110499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinkauf H., von Döhren H. Biosynthesis of peptide antibiotics. Annu Rev Microbiol. 1987;41:259–289. doi: 10.1146/annurev.mi.41.100187.001355. [DOI] [PubMed] [Google Scholar]
- Liesch J. M., Millington D. S., Pandey R. C., Rinehart K. L., Jr Berninamycin. 2. Products of acidic hydrolysis, methanolysis, and acetolysis of berninamycin A1. J Am Chem Soc. 1976 Dec 8;98(25):8237–8249. doi: 10.1021/ja00441a058. [DOI] [PubMed] [Google Scholar]
- Mihara H., Aoyagi H., Lee S., Waki M., Kato T., Izumiya N. Cyclic peptides. XVI. Syntheses of AM-toxin I analogs containing a lower or higher homolog of the component L-2-amino-5-(p-methoxyphenyl)pentanoic acid residue. Int J Pept Protein Res. 1984 May;23(5):447–453. [PubMed] [Google Scholar]
- Morris S. L., Hansen J. N. Inhibition of Bacillus cereus spore outgrowth by covalent modification of a sulfhydryl group by nitrosothiol and iodoacetate. J Bacteriol. 1981 Nov;148(2):465–471. doi: 10.1128/jb.148.2.465-471.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris S. L., Walsh R. C., Hansen J. N. Identification and characterization of some bacterial membrane sulfhydryl groups which are targets of bacteriostatic and antibiotic action. J Biol Chem. 1984 Nov 10;259(21):13590–13594. [PubMed] [Google Scholar]
- Pitner T. P., Urry D. W. Conformational studies of polypeptide antibiotics. Proton magnetic resonance of stendomycin. Biochemistry. 1972 Oct 24;11(22):4132–4137. doi: 10.1021/bi00772a016. [DOI] [PubMed] [Google Scholar]
- Ruhr E., Sahl H. G. Mode of action of the peptide antibiotic nisin and influence on the membrane potential of whole cells and on cytoplasmic and artificial membrane vesicles. Antimicrob Agents Chemother. 1985 May;27(5):841–845. doi: 10.1128/aac.27.5.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzeszotarska B., Kubica Z., Tarnawski J. O wpływie reszt alpha, beta-dehydroaminokwasowych na aktywnoś biologiczna peptydów. Postepy Biochem. 1987;33(4):533–560. [PubMed] [Google Scholar]
- Schmidt U., Häusler J., Ohler E., Poisel H. Dehydroamino acids, alpha-hydroxy-alpha-amino acids and alpha-mercapto-alpha-amino acids. Fortschr Chem Org Naturst. 1979;37:251–251. doi: 10.1007/978-3-7091-8545-2_3. [DOI] [PubMed] [Google Scholar]
- Sharma A. K., Chauhan V. S. Analogues of luteinizing hormone-releasing hormone (LH-RH) containing dehydroalanine in 6th position. Int J Pept Protein Res. 1988 Feb;31(2):225–230. doi: 10.1111/j.1399-3011.1988.tb00027.x. [DOI] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]



