Abstract
Tissue growth and organ size are determined by coordinated cell proliferation and apoptosis in development. Recent studies have demonstrated that Hippo (Hpo) signaling plays a crucial role in coordinating these processes by restricting cell proliferation and promoting apoptosis. Here we provide evidence that the Mob as tumor suppressor protein, Mats, functions as a key component of the Hpo signaling pathway. We found that Mats associates with Hpo in a protein complex and is a target of the Hpo serine/threonine protein kinase. Mats phosphorylation by Hpo increases its affinity with Warts (Wts)/large tumor suppressor (Lats) serine/threonine protein kinase and ability to upregulate Wts catalytic activity to target downstream molecules such as Yorkie (Yki). Consistently, our epistatic analysis suggests that mats acts downstream of hpo. Coexpression analysis indicated that Mats can indeed potentiate Hpo-mediated growth inhibition in vivo. Our results support a model in which Mats is activated by Hpo through phosphorylation for growth inhibition, and this regulatory mechanism is conserved from flies to mammals.
Keywords: Drosophila, growth inhibition and tumor suppression, hippo, mats, wts
Introduction
Tissue growth and organ size are determined by coordinated cell proliferation and apoptosis in development. Recent studies have identified a new growth-inhibitory tumor suppression pathway that coordinates cell proliferation and apoptosis during Drosophila development (Hipfner and Cohen, 2004; Edgar, 2006; Hariharan and Bilder, 2006). Two protein kinases Hippo (Hpo) (Harvey et al, 2003; Jia et al, 2003; Pantalacci et al, 2003; Udan et al, 2003; Wu et al, 2003) and Warts (Wts)/large tumor suppressor (Lats) (Justice et al, 1995; Xu et al, 1995), and a scaffold protein Salvador (Sav)/Shar-pei (Kango-Singh et al, 2002; Tapon et al, 2002), are key components of this pathway. Moreover, two FERM-domain proteins, Merlin (Mer) and Expanded (Ex), function upstream of Hpo (Hamaratoglu et al, 2006), and Mob as tumor suppressor (Mats), associates with Wts to stimulate the catalytic activity of the Wts protein kinase (Lai et al, 2005). Recently, both putative receptor and ligand that function further upstream of, or in parallel with, Hpo signaling have been identified (reviewed in Hariharan, 2006). A major signal output of this growth inhibitory pathway is to inactivate a transcription coactivator Yorkie (Yki) via phosphorylation by Wts kinase (Huang et al, 2005). In addition to Cyclin E and Drosophila inhibitor of apoptosis 1 (diap1), the bantam microRNA is also found to be a target of the Hpo pathway (Nolo et al, 2006; Thompson and Cohen, 2006). Most components in this emerging signaling pathway are conserved from yeast to flies and humans, suggesting that this pathway plays a fundamental role in cellular regulation.
The function of Mob proteins has been better studied in yeast, Drosophila and mammalian cells, which revealed a conserved property of Mob proteins as a binding partner as well as a coactivator of protein kinases of the Ndr (nuclear Dbf2-related) family (Hergovich et al, 2006b). As stated above, Drosophila Mats/dMob1 is required for mediating Hpo signaling by regulating Wts kinase activity in growth inhibition and tumor suppression (Lai et al, 2005). All four Drosophila mob genes dMob1–4 genetically interact with trc (tricornered) (He et al, 2005a), the fly Ndr homolog important for maintaining integrity of epidermal outgrowths and regulating dentritic tiling and branching (Emoto et al, 2004; He et al, 2005b). In the budding yeast Saccharomyces cerevisiae, Mob1 binds to and activates Dbf2/Dbf20 protein kinases for controlling mitotic exit and cytokinesis (Komarnitsky et al, 1998; Lee et al, 2001; Mah et al, 2001). Similarly, Mob1 is required for the activation of Sid2, an Ndr family kinase in the fission yeast Schizosaccharomyces pombe essential for cytokinesis (Hou et al, 2000, 2004). In human, hLats1 preferentially interacts with hMob1/hMats, but not hMob2 protein, and appeared to be required for promoting mitotic exit (Bothos et al, 2005), as well as cytokinesis (Yang et al, 2004). Importantly, the function of Mob proteins has been highly conserved in evolution. For instance, the human Mob1A/Mats1 protein has been shown to act as a kinase activator and can rescue the lethality and tumor phenotypes of Drosophila mats mutants (Lai et al, 2005).
Structural analysis of a human Mob1 protein, Mob1A/Mats1, revealed several important features of Mob family proteins (Stavridi et al, 2003). One is that several highly conserved residues are responsible for generating an atypical Cys2-His2 zinc-binding site, which is predicted to contribute to the stability of the Mob protein. Another striking feature is that there is a flat surface rich in acidic residues on one side of the protein. This property provides the structural basis for a Mob protein to interact with its partner, such as Ndr family kinases through electrostatic forces. Indeed, a 65-amino-acid region rich in basic residues exists in the N-terminal side of the kinase domain of Ndr family kinases, and alterations in the basic residues can prevent the kinases from binding to Mob proteins (Bichsel et al, 2004; Bothos et al, 2005; Hergovich et al, 2006b). Finally, hMob1A adopts a globular structure involving residues throughout the polypeptide. Mob proteins are small and usually do not carry any other structural motifs other than the Mob domain.
Although previous studies suggest that Ndr family kinases can be activated by upstream regulators such as Cdc15, Hpo and Mst kinases via phosphorylation in yeast, flies or human cells (Lee et al, 2001; Mah et al, 2001; Wu et al, 2003; Hou et al, 2004; Chan et al, 2005; Hergovich et al, 2006b), very little is known about how Mob is regulated. Studies carried out in yeast and mammalian cells suggested that Mob proteins may be regulated through phosphorylation. For instance, yeast Mob1 was shown to be essential for the phosphorylation of Dbf2 by an upstream protein kinase Cdc15 and Mob1 itself was also phosphorylated by Cdc15 (Mah et al, 2001). However, the functional significance of this modification has not been elucidated. Work on human Mob1A/Mats1 also suggested that phosphorylation might provide a mechanism for regulating hMob1A activity (Bichsel et al, 2004). In this study, we have tested a hypothesis that Mats is directly activated by Hpo kinase to regulate Wts kinase activity for growth inhibition and tumor suppression. Using the Drosophila system, we found that Mats can be complexed with Hpo and is a target of the Hpo protein kinase. Similarly, human Mats1 is also a target protein of mammalian Mst kinases. Mats phosphorylation by Hpo increases its affinity with Wts protein kinase and ability to increase Wts activity to target Yki. Moreover, our epistatic analysis suggested that mats acts downstream of hpo. Genetic analysis indicated that Mats functions together with Hpo for mediating growth inhibition of developing organs. Therefore, the Mob as tumor suppressor protein, Mats, functions as a critical component of the Hpo signaling pathway. Our results support a model in which Mats is activated by Hpo through phosphorylation for growth inhibition, and this regulatory mechanism is conserved from flies to mammals.
Results
Genetic interactions between mats, hpo, wts and sav
We have previously shown that Mats functions as a coactivator of the Wts protein kinase to negatively regulate tissue growth (Lai et al, 2005). To further test the idea that Mats is a key component of the Hpo signaling pathway, we carried out genetic analysis using an in vivo assay in which a small-eye phenotype was induced by overexpression of a full-length Hpo or an N-terminal fragment of Hpo (HpoN), a constitutively activated form of Hpo (Jia et al, 2003; Udan et al, 2003) (Figure 1B and C, respectively). A wild-type eye is shown in Figure 1A for comparison. Whereas mats overexpression did not cause any morphological defects in the eye (Figure 1E; Lai et al, 2005), the Hpo- and HpoN-induced small-eye phenotype was strongly enhanced by coexpression with mats (Figure 1F and G), suggesting that Mats and Hpo synergistically interact to restrict tissue growth. Overexpression of a dominant-negative form of Hpo, HpoC (C-terminal fragment of Hpo) (Jia et al, 2003), did not cause obvious defects in the eye (Figure 1D). Similarly, flies that expressed both Mats and HpoC exhibited a normal eye phenotype (Figure 1H).
Figure 1.
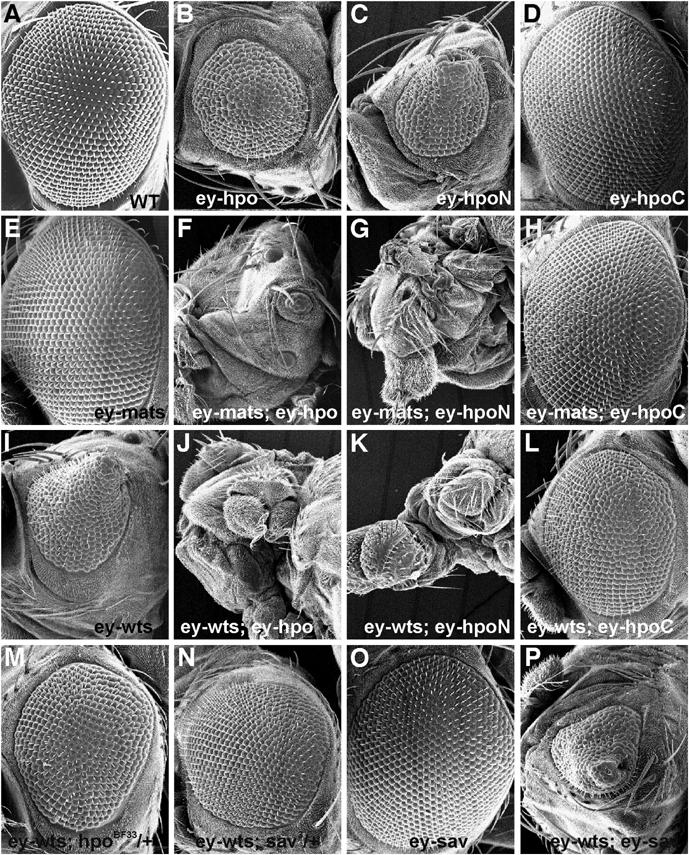
Genetic interactions between Hpo pathway components hpo, sav, wts and mats. Scanning electron microscopy (SEM) images of adult compound eyes are shown. (A) Wild type. (B) In ey-Gal4/UAS-hpo flies, overexpression of Hpo in the developing eye reduced eye size. (C) An N-terminal fragment of Hpo (HpoN, a constitutively active form) in ey-Gal4/UAS-hpoN flies can also reduce eye size. (D) Overexpression of a C-terminal fragment of Hpo (HpoC, a putative dominant-negative form) in ey-Gal4/UAS-hpoC flies had no obvious effect on eye morphology. (E) The eye of ey-Gal4/UAS-mats flies was morphologically normal. Coexpression of Mats with Hpo in the eye of ey-Gal4/UAS-hpo; +/UAS-mats flies (F) or HpoN in ey-Gal4/UAS-hpoN; +/UAS-mats flies (G) markedly enhanced the small eye phenotype so that the eye became much smaller or abolished. (H) Coexpression of Mats with HpoC in ey-Gal4/UAS-hpoC; +/UAS-mats flies did not cause any obvious defect in the eye. (I) Overexpression of Wts in ey-Gal4/UAS-Myc-wts flies by using a strong transgenic line 6R reduced eye size and caused the eye to have a cone-like shape. Coexpression of wts with hpo in ey-Gal4/UAS-Myc-wts UAS-hpo flies (J) or hpoN in ey-Gal4/UAS-Myc-wts UAS-hpoN flies (K) completely abolished eye development. In contrast, the small and cone-like eye phenotypes induced by Wts were suppressed by HpoC coexpression in ey-Gal4/UAS-Myc-wts UAS-hpoC flies (L) or by reduction of endogenous activity of hpo in ey-Gal4/UAS-Myc-wts; +/hpoBF33 flies or sav in ey-Gal4/UAS-Myc-wts; +/sav3 flies (M, N, respectively). (O) Overexpression of Sav in ey-Gal4/UAS-HA-sav flies did not disrupt eye formation. However, Sav was able to enhance the small eye phenotype caused by Wts in ey-Gal4/UAS-Myc-wts UAS-HA-sav flies (P). Anterior is to the left in all panels.
Genetic interactions between wts, hpo and sav were also examined using the eye system. Whereas overexpression of Wts in developing eye using a strong P[UAS-myc-wts]6R transgenic line significantly reduced the eye size and caused a cone-eye phenotype (Figure 1I), coexpression of Wts with Hpo or HpoN abolished eye development (Figure 1J and K, respectively). On the other hand, Wts-induced small cone-eye phenotype was suppressed by HpoC coexpression (Figure 1L), as well as by the reduction of the endogenous hpo or sav function (Figure 1M and N, respectively). Although overexpression of Sav in a wild-type background did not cause any morphological defect in the eye (Figure 1O), Wts-induced small-eye phenotype can be further enhanced by Sav coexpression (Figure 1P). Altogether, these results are consistent with a previously established model in which Wts functions downstream of Hpo and Sav to control tissue size.
Epistatic analysis suggests that mats acts downstream of hpo
To investigate epistatic relationship between mats and hpo, mats mutant cells that simultaneously overexpressed hpo were generated using the MARCM technique. As shown previously, loss of mats function in mosaic tissues such as larval eye discs caused tissue overgrowth (Figure 2B; Lai et al, 2005), compared to eye discs containing wild-type clones (Figure 2A). In contrast, overexpression of hpo in MARCM clones was sufficient to inhibit tissue growth (Figure 2C). Cells overexpressing hpo in the absence of endogenous mats function exhibited tissue overgrowth phenotype similar to that of mats single mutant (compare Figure 2D with B). Therefore, mats is epistatic to hpo and may act downstream of hpo. In another epistatic analysis, hpoN was expressed in all cells posterior to the morphogenetic furrow in the developing eye to induce apoptosis (Figure 2E). Using this assay, we found that the ability of hpoN to promote apoptosis was mostly dependent on mats (Figure 2F–H). By counting the number of TUNEL-positive spots in the mosaic larval eye discs (n=7), the percentages of apoptotic cells in mats wild-type and mutant tissues were estimated. Over 85% of the apoptotic cells were identified in mats wild-type tissues, whereas much fewer apoptotic cells (15%) were detected in mats mutant tissues that were about of the same size as neighboring wild-type areas. The fact that residual apoptosis could still be induced by Hpo in the absence of mats indicates that Hpo could act through other targets in addition to Mats to induce apoptosis. These results are consistent with the idea that mats functions downstream of hpo.
Figure 2.
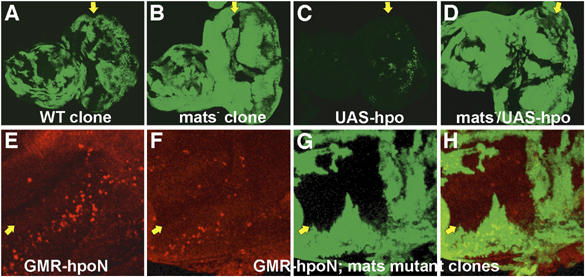
mats is epistatic to hpo. Third instar larval eye imaginal discs are shown. Clones in mosaic eye discs were positively marked by GFP (green) in (A–D) using the MARCM method (Lee and Luo, 1999), and mats mutant clones were marked by the absence of β-galactosidase (green) in (F–H). (A) An eye disc that contains GFP-labeled wild-type MARCM clones is shown as a control. The genotype is w ey-FLP UAS-GFP/+; Tub-Gal4 FRT82B Tub-Gal80/FRT82B P[w+]90E. (B) An eye disc that contains GFP-labeled mats mutant MARCM clones exhibited an overgrowth phenotype. The genotype is w ey-FLP UAS-GFP/+; Tub-Gal4 FRT82B Tub-Gal80/FRT82B matse235. (C) Overexpression of Hpo in wild-type MARCM clones markedly inhibited tissue growth. The genotype is w ey-FLP UAS-GFP/+; UAS-hpo/+; Tub-Gal4 FRT82B Tub-Gal80/FRT82B P[w+]90E. (D) Loss of mats function effectively suppressed the growth inhibitory effect of Hpo. The genotype is w ey-FLP UAS-GFP/+; +/UAS-hpo; Tub-Gal4 FRT82B Tub-Gal80/FRT82B matse235. (E) GMR-Gal4 UAS-hpoN/+ and (F–H) w ey-FLP/+; +/GMR-Gal4 UAS-hpoN; FRT82B arm-lacZ/FRT82B matse235 eye discs were used for TUNEL staining of apoptotic cells (red). When driven by GMR-Gal4, HpoN was sufficient to cause apoptosis in the region posterior to the morphogenetic furrow (MF) in eye discs (E), and this HpoN-induced apoptosis occurred mostly in cells that were wild type for mats (F–H). Anterior is to the left in (A–D) and up-left in (E–H). Yellow arrows identify the morphogenetic furrow.
Mats is a substrate of Hpo protein kinase in vitro
In S. cerevisiae, a Mob family protein Mob1 acts downstream of the yeast homolog of Hpo protein kinase Cdc15 and is required for the function of Dbf2 protein kinase, the yeast homolog of Wts/Lats proteins (Lee et al, 2001; Mah et al, 2001). In addition to phosphorylating Dbf2 to activate its Mob1-dependent kinase activity, Cdc15 also phosphorylates Mob1, suggesting that Mob1 might be directly regulated by Cdc15 kinase (Mah et al, 2001). Based on the data from yeast and Drosophila, we hypothesized that Mats is directly regulated by Hpo kinase via phosphorylation. To test this hypothesis, Flag-tagged Hpo kinase protein was expressed in transiently transfected fly S2 cells or human embryonic kidney (HEK) 293T cells, and immunoprecipitated for analysis of its catalytic activity. Bacterially produced GST-Mats or GST proteins were used as substrates. In the presence of [γ-32P]ATP, substantial incorporation of 32P into GST-Mats was observed following incubation with Hpo protein (Figure 3A, lane 3). Mats phosphorylation was unlikely to be caused by any other contaminating kinases because Mats was not phosphorylated when HpoKD protein was used (Figure 3A, lane 4). GST served as a negative control (Figure 3A, lane 2). Interestingly, Sav did not appear to facilitate Hpo to phosphorylate Mats although it slightly increased Hpo's activity to autophosphorylate (Figure 3A, compare lane 5 with lane 3). Sav has been shown to be able to facilitate Hpo to phosphorylate Wts (Wu et al, 2003). Thus, Hpo may use a different mechanism to target Mats. Moreover, we found that Hpo phosphorylated human Mats1 (Figure 3B), and a rat Hpo ortholog Mst2 phosphorylated not only hMats1 (data not shown), but also fly Mats (Figure 3C). These results indicate that Mats is a target of Hpo/Mst, and the activity of Hpo/Mst kinases to phosphorylate Mats proteins is evolutionarily conserved.
Figure 3.
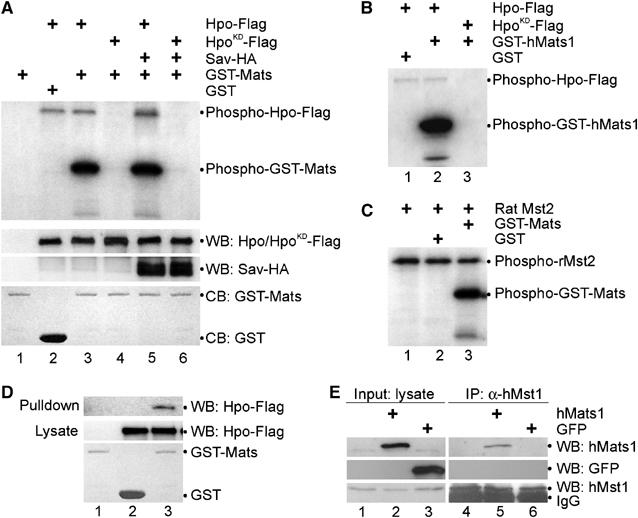
Mats associates with Hpo and is phosphorylated by Hpo protein kinase. (A) Hpo specifically phosphorylates Mats in vitro. pAc-hpo-Flag, pAc-hpoKD-Flag and pAc-sav-HA were transfected into S2 cells as indicated. Lysates of transfected cells were used for Western blot (WB) to show the inputs (middle two panels). Anti-Flag antibody was used to immunoprecipitate (IP) Hpo-Flag or HpoKD-Flag proteins for an in vitro kinase assay, in which GST-Mats or GST proteins were used as substrates (the bottom panel; CB: Coomassie blue staining). Hpo autophosphorylation and Mats phosphorylation by Hpo/HpoKD are shown in the top panel. (B) GST was not a substrate of Hpo kinase (lane 1). Hpo (lane 2) but not HpoKD (lane 3) can phosphorylate human Mats1 in vitro. (C) A rat Hpo ortholog, Mst2 kinase, can phosphorylate GST-Mats (lane 3) but not GST (lane 2) in vitro. Like Hpo, Mst2 mediates autophosphorylation (lanes 1–3). (D) In an in vitro pull-down assay, Mats was shown to associate with Hpo. Bacterially produced GST-Mats (lanes 1 and 3) or GST (lane 2) proteins were tested for their ability to interact with Hpo-Flag (lanes 2 and 3) produced in transfected S2 cells. (E) Human Mats1 was co-immunoprecipitated with the endogenous human Mst1. pCMV-hMats1 and pCMV-GFP were transfected into HEK293T cells as indicated. Inputs of hMats1 (lane 2), GFP (lane 3) and hMst1 (lanes 1–3) proteins are shown in panels on the left. Immunoprecipitations were carried out using anti-hMst1 antibodies to isolate the endogenous hMst1 protein (lanes 4-6, the bottom-right panel), and hMats1 (lane 5) but not GFP (lane 6) was co-immunoiprecipitated (top two panels on the right).
Hpo and Mats associate to form a protein complex
To address whether Mats directly interacts with the Hpo kinase, GST-Mats fusion protein was incubated with cell lysate that contained Flag-tagged Hpo protein. Hpo can be pulled down by GST-Mats but not GST, indicating that Mats was involved in the interaction with Hpo (Figure 3D). In co-immunoprecipitation experiments, Mats was shown to be associated with Hpo kinase when expressed in HEK293T cells, and Mats can also interact with the endogenous human Mst1 (hMst1) protein in living cells (data not shown). Moreover, the fly Hpo protein was found to be able to associate with the endogenous hMats protein in HEK293T cells (data not shown). Similarly, human Mats1 can also be associated with the endogenous hMst1 kinase protein (Figure 3E, lanes 2 and 5; data not shown). As a negative control, GFP was not precipitated under the same conditions (Figure 3E, lanes 3 and 6). Of note, Mats–Hpo/Mst interaction was weaker compared to Mats–Wts protein interaction. Interestingly, hMst1 did not appear to co-exist in the Wts/Mats protein complex in transfected cells (Lai et al, 2005; data not shown). Thus, Mats protein is able to specifically associate with Hpo/Mst, which would allow Hpo/Mst kinases to be more effective in phosphorylating Mats. This mechanism of Mats regulation by Hpo/Mst kinases is conserved from flies to humans.
Hpo phosphorylation activates Mats to upregulate Wts protein kinase
To reveal the significance of Hpo-mediated phosphorylation of Mats, we have devised a functional assay to measure the ability of Mats to stimulate Wts kinase activity with or without Hpo phosphorylation. To begin with, bacterially produced GST-Mats protein was treated with immobilized Hpo-Flag or HpoKD-Flag, and then collected from supernatant. As shown in a control experiment, the supernatant contained only phosphorylated Mats, no detectable Hpo kinase (Figure 4A, lane 7). Thus, Hpo kinase was unlikely responsible for the subsequent phosphorylation reaction. After Hpo-mediated phosphorylation using cold ATP, Mats protein was incubated with Wts kinase in the presence of radioactive [γ-32P]ATP. Wts kinase activity was measured by the incorporation of 32P into Yki, a substrate of Wts kinase (Huang et al, 2005). Wts activity was hardly detected in the absence of Mats (Figure 4A, lanes 1) or when HpoKD-treated Mats was added (Figure 4A, lane 3). Hpo phosphorylation, however, increased the ability of Mats to stimulate the catalytic activity of Wts kinase (Figure 4A, lane 2). Similar observations were also made with the human Mats1 protein when it was tested in this in vitro kinase assay (data not shown). Moreover, Yki was not phosphorylated when WtsKD was tested in this assay (Figure 4A, lanes 4–6), suggesting that Wts was responsible for Yki phosphorylation. Thus, phosphorylation of Mats by Hpo makes Mats more potent to activate Wts kinase, and this Hpo-mediated activation of Mats is evolutionarily conserved.
Figure 4.
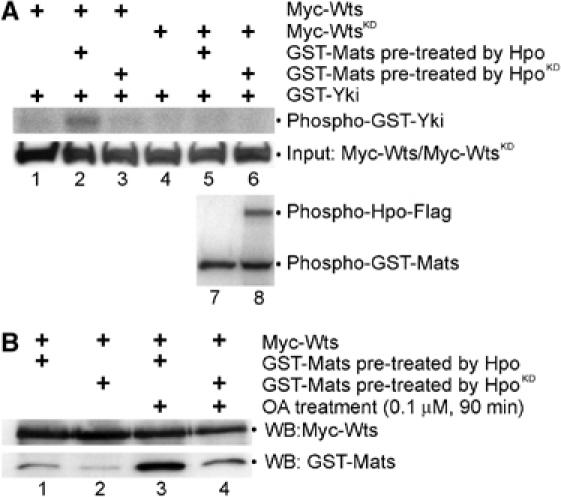
Hpo phosphorylation of Mats increases the affinity between Mats and Wts and the ability of Mats to activate Wts. (A) Equal amounts of bacterially produced GST-Mats were preincubated for cold phosphorylation with Hpo or HpoKD immobilized on agarose beads, and then collected from supernatant and mixed with Myc-Wts for in vitro kinase reactions using [γ-32P]ATP. Myc-Wts was produced in transfected HEK293T cells, which were not treated with OA. GST-Yki was used as a substrate of Wts kinase. To ensure that immobilized Hpo kinase proteins were not carried over to the subsequent Wts-mediated phosphorylation reaction, [γ-32P]ATP was added in the Hpo-mediated kinase reaction. Whereas phospho-GST-Mats was detected in both supernatant and pellet (lanes 7 and 8), Hpo protein was exclusively found only in the pellet (lane 8). Thus, phosphorylation of Yki (lane 2) must be mediated by Wts kinase. Indeed, Yki phosphorylation was not detectable when WtsKD was used (lane 5). (B) Phosphorylation of Mats by Hpo increases the affinity between Wts and Mats proteins. Equal amounts of Myc-Wts produced in HEK293T cells treated without (lanes 1 and 2) or with (lanes 3 and 4) OA were linked to anti-Myc agarose beads, and GST-Mats treated with Hpo (lanes 1 and 3) or HpoKD (lanes 2 and 4) was tested for their ability to associate with Wts.
Hpo phosphorylated Mats displays increased affinity to Wts protein kinase
Hpo phosphorylation of Mats makes Mats a more potent activator of Wts kinase, which might be partly caused by an increased affinity between Wts and Hpo phosphorylated Mats. To test this, Mats protein was treated with Hpo kinase or HpoKD in the presence of cold ATP. An equal amount of Wts protein produced in transfected HEK293T cells was used in in vitro protein-binding analysis. The amount of Wts and Mats proteins derived from Wts–Mats complexes was examined by Western blot analysis. As expected, we found that more Hpo-treated Mats proteins were detected in Wts–Mats complexes than in those treated with HpoKD (Figure 4B, compare lane 1 with lane 2, and lane 3 with lane 4). Thus, phosphorylation of Mats by Hpo kinase increases the affinity between Mats and Wts. Moreover, Wts protein derived from okadaic acid (OA)-treated cells exhibited an increased affinity to Mats (Figure 4B, compare lane 3 with lane 1, and lane 4 with lane 2), suggesting that Wts phosphorylation may play a role in increasing its affinity to Mats.
Wts protein kinase is activated by Mats in living cells
To investigate how Wts kinase is regulated in living cells, Wts protein was expressed in HEK293T cells under various conditions and immunoprecipitated for analysis of its kinase activity to autophosphorylate and phosphorylate Mats. Whereas Wts protein isolated from transfected cells in the absence of OA treatment did not display any detectable kinase activity with or without Mats overexpression (Figure 5A, lanes 1 and 2), treatment of wts-transfected cells with OA markedly increased Wts kinase activity, by 17-fold (Figure 5A, compare lane 3 with lane 1). Coexpression of Wts with Mats in OA-treated cells further increased Wts kinase activity four-fold (Figure 5A, compare lane 4 with lane 3). Clearly, this included the effect of Mats on Wts production and/or stability as Mats increased the steady levels of Wts protein (Figure 5A, the third and fifth panels from the top). After normalizing the amount of Myc-Wts protein used in the kinase reactions, Mats was found to be able to increase Wts kinase activity by two-fold (Figure 5A, compare lane 4 with lane 3, the top and third panels). Similar results were observed using GST-Yki as a substrate (Figure 5C and data not shown). Thus, Mats is a coactivator of Wts kinase for phosphorylation of targets such as Yki. In fly S2 cells, Wts kinase activity was increased by Mats with or without OA treatment (data not shown), suggesting that Wts and Mats are not normally inhibited by protein phosphatases in this cell type. Consistent with this notion, Hpo kinase is active in S2 cells in the absence of OA treatment (Figure 3A and B), to constitutively activate Wts. As OA functions to block protein phosphatases, such as PP2A, our results suggest that Mats and/or Wts phosphorylation are critical for Wts kinase activation in living cells.
Figure 5.
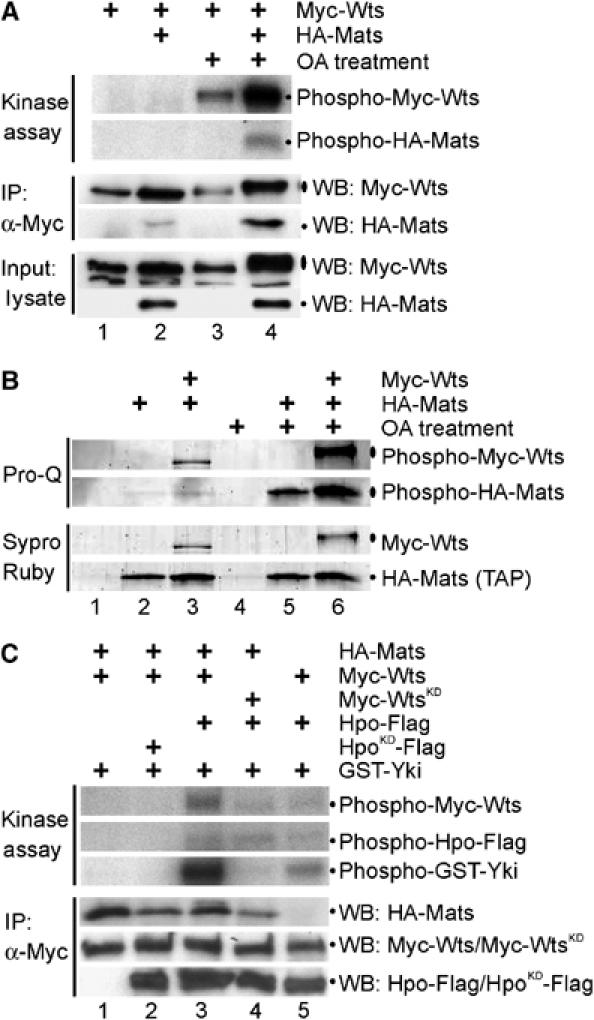
Mats is a phosphoprotein and Mats is critical for Hpo-mediated Wts activation in living cells. (A) OA treatment as well as coexpression of Mats increased the catalytic activity of Wts kinase as shown in an in vitro kinase assay. Equal amounts of Myc-wts and HA-mats DNA were transfected into HEK293T cells. Myc-Wts was immunoprecipitated from cells that were cotransfected without (lanes 1 and 3) or with (lanes 2 and 4) HA-mats in the absence (lanes 1 and 2) or presence (lanes 3 and 4) of OA treatment as indicated. (B) Both Mats and Wts are phosphoproteins in living cells. Using a TAP method, the HA-Mats protein complex was highly purified and examined by SDS–PAGE analysis. Sypro Ruby staining was used to detect all proteins that include HA-Mats and other proteins in the HA-Mats complex. Pro-Q staining identified phosphoproteins. HEK293T cells were either single or double-transfected with or without OA treatment as indicated. (C) Hpo activates Wts kinase and Mats is critical for Hpo-mediated Wts activation in living cells. HEK293T cells were either double or triple-transfected as indicated. After immunoprecipitating Myc-Wts or Myc-WtsKD protein complex using anti-Myc antibodies, HA-Mats, Myc-Wts/Myc-WtsKD and Hpo-Flag/HpoKD-Flag proteins were examined by Western blot analysis. Equal amounts of Myc-Wts/Myc-WtsKD proteins were used for in vitro kinase reactions, and catalytic activities of Wts and Hpo for autophosphorylation were measured. Moreover, bacterially produced GST-Yki protein was added to determine Wts kinase activity. Hpo does not appear to phosphorylate Yki (lane 4 and data not shown). The cells were not treated with OA.
Mats and Wts are phosphoproteins in living cells
To determine the effect of OA treatment on Wts and Mats phosphorylation, Mats was tagged and the Mats protein complex was isolated by a tandem affinity purification (TAP) method. Phosphorylation state of Mats and Wts proteins was evaluated by Pro-Q staining, which can selectively stain phosphorylated proteins. Without OA treatment, Mats phosphorylation was low, whereas Wts was only moderately phosphorylated (Figure 5B, lanes 2 and 3). However, OA treatment increased phosphorylation of both Mats and Wts proteins (Figure 5B, lanes 5 and 6). Thus, both Mats and Wts are phosphoproteins, and their phosphorylation in living cells can be increased by inhibition of protein phosphatases.
OA has been shown to cause activation of the human Hpo orthologs Mst kinases (e.g., Lee and Yonehara, 2002; O'Neill et al, 2004), which might contribute to the phosphorylation and activation of Mats/Wts proteins (Figure 5A and B). If this is the case, Hpo/Mst kinases are expected to be sufficient to activate Mats and Wts proteins in cells without OA treatment. Experiments described below have been conducted to test this idea.
Wts is activated by Hpo protein kinase, and Mats is critical for Hpo-mediated Wts activation in living cells
To test the ability of Hpo to activate Wts kinase in living cells, Myc-Wts was expressed in HEK293T cells and immunoprecipitated for in vitro kinase reactions. Wts was tested for its ability to autophosphorylate and to phosphorylate a downstream target Yki. In the absence of OA treatment, Wts exhibited no detectable kinase activity even in the presence of Mats (Figure 5C, lane 1; also see Figure 5A, lane 2). However, coexpression of Hpo stimulated Wts kinase activity (Figure 5C, lane 3), and the kinase activity of Hpo was clearly required for mediating Wts activiation (Figure 5C, lane 2). Consistent with previous findings (reviewed in Edgar, 2006), Hpo associated with Wts and consequently Hpo was co-immunoprecipitated. Phosphorylation of Hpo protein occurred in the presence of Wts or WtsKD (Figure 5C, lane 3 and lane 4, respectively), suggesting that Hpo, but not Wts, was responsible for Hpo phosphorylation. Although Wts was phosphorylated by Hpo to a certain extent (Figure 5C, lane 4, top panel), Wts autophosphorylation contributed to the increase in the level of phospho-Wts protein when coexpressed with Hpo (Figure 5C, compare lane 3 with lane 4, top panel).
To investigate the importance of Mats in Hpo-mediated activation of Wts kinase, Wts and Hpo were coexpressed in the absence of Mats. Compared to a control in which all three proteins were coexpressed (Figure 5C, lane 3), Wts kinase activity was apparently reduced when Mats was not coexpressed (Figure 5C, lane 5). Therefore, Mats plays a critical role in Hpo-mediated Wts activation, and this finding is consistent with our model that Hpo activates Mats via phosphorylation so that Mats becomes a more potent activator of Wts kinase (Figure 6).
Figure 6.
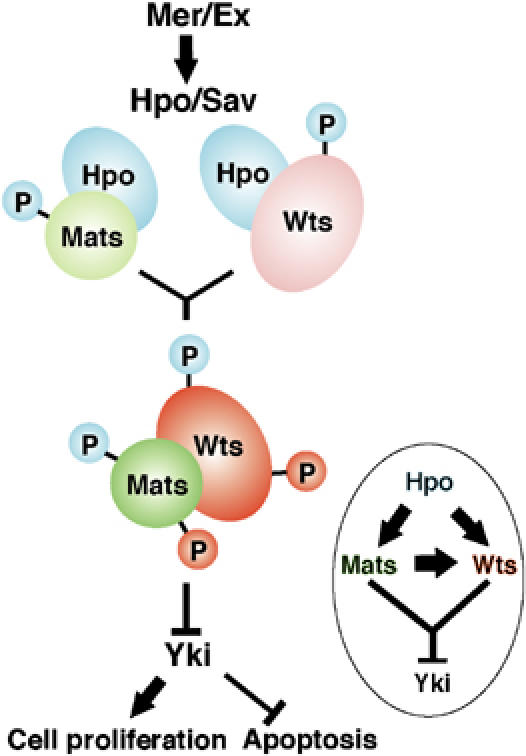
A model for growth inhibition by Hpo, Wts and Mats tumor suppressors. Hpo kinase, acting downstream of Mer and Ex, directly activates Mats via phosphorylation in addition to targeting Wts. Consequently, Mats becomes a potent activator of Wts kinase to phosphorylate a downstream transcription cofactor Yki for down-regulation. Hpo-mediated phosphorylation is indicated by small blue circle, and Wts-mediated phosphorylation by small red circles.
Discussion
Recent studies have defined an emerging growth inhibitory pathway mediated by Fat, Mer/Ex, Hpo/Sav and Wts/Mats proteins in tissue growth and organ size control in Drosophila (Hipfner and Cohen, 2004; Edgar, 2006; Hariharan and Bilder, 2006; Hariharan, 2006). In our previous work, we have shown that Mats functions as a coactivator of the Wts protein kinase (Lai et al, 2005). In this study, we have focused on addressing how Mats is activated to regulate Wts kinase activity. Our genetic analysis suggests that Mats acts downstream of Hpo and is a critical component of the Hpo signaling pathway. Moreover, we provide evidence that Hpo-mediated phosphorylation increases Mats's activity as a coactivator of the Wts protein kinase, and this regulatory mechanism is conserved from flies to humans. Therefore, Hpo-mediated phosphorylation of Mats significantly contributes to Wts activation. In a simple model (Figure 6), Hpo needs to directly phosphorylate Wts as well as Mats in order for Wts kinase to be fully activated. Although both Wts and Mats are activated by Hpo-mediated phosphorylation, further investigations are needed to address how Hpo phosphorylation and Mats binding are coordinated for Wts activation.
Phosphorylation of Mob family proteins
In this report, we provide evidence that Mats is a target of Hpo/Mst protein kinases and Hpo/Mst-mediated phosphorylation positively regulates Mats protein's coactivator activity for Wts protein kinase. Importantly, we found that Mats exists as a phosphoprotein in living cells, indicating that Mats phosphorylation occurs under physiological conditions. In addition to Hpo/Mst, Wts kinase has also been shown to target Mats for phosphorylation (Lai et al, 2005), although the physiological effect of this modification has not been elucidated. In S. cerevisiae, the founding member of the Mob superfamily Mob1 was found to be a phosphoprotein and a substrate for the Mps1 kinase. Mob1 is also phosphorylated by an upstream regulator Cdc15 kinase (Mah et al, 2001). However, the role of Cdc15 in Mob1 phosphorylation has not been revealed even though Mob1 is known to be required for Cdc15-mediated activation of its binding partner Dbf2 kinase (Lee et al, 2001; Mah et al, 2001). In mammalian cells, protein phosphatase 2A inhibition by OA treatment caused phosphorylation of a Mob family protein (Moreno et al, 2001). Moreover, OA-induced modification on hMob1 was shown to be critical for its binding to its partner Ndr kinase (Bichsel et al, 2004). Thus, phosphorylation appears to be a common mechanism for Mob regulation.
Mats is activated by Hpo-mediated phosphorylation
Consistent with the finding that Mats is activated by Hpo via phosphorylation for upregulating Wts kinase activity, our epistatic analysis suggests that Mats is acting downstream of Hpo. To our knowledge, this is the first case that Ste20 family protein kinase-mediated phosphorylation of Mob is critical for regulating the catalytic activity of Ndr family protein kinase such as Wts. At this point, it is not clear how Mob proteins function to activate Ndr family kinases. Based on the results from recent studies of human Mob1 and Ndr family kinases, a potential mechanism is that Ndr family kinase is rapidly recruited by hMob1 to the plasma membrane for activation (Hergovich et al, 2005, 2006a). We speculate that Hpo phosphorylation might facilitate Mats to associate to the membrane through an unknown mechanism, which in turn recruits Wts to the membrane as Hpo phosphorylated Mats has an increased affinity to Wts. Subsequently, Wts is activated by phosphorylations mediated by protein kinases such as Hpo. Mats as a target of Hpo kinase, is able to associate with Hpo in a protein complex. As Hpo/Mst1 kinase was not present in the Mats/Wts protein complex (Lai et al, 2005; data not shown), it appears that Mats simultaneously cannot associate with Hpo and Wts in the same protein complex.
In addition to this membrane recruitment model described above, our data also support an active and more direct role of Mats in upregulating Wts kinase. From our in vitro kinase assays, we found that Hpo-mediated phosphorylation increases the affinity between Mats and Wts, as well as the ability of Mats to activate Wts kinase activity in the absence of any membrane structures. Our results support a model in which Mats binding likely causes a conformational change of Wts for Wts activation. In the case of human Ndr kinase, an autoinhibitory effect of hNdr can be released by hMob1 binding (Bichsel et al, 2004), which presumably induces a conformational change in hNdr for its activation. Finally, we found that Mats increases the steady level of Wts protein, which contributes to the increase in Wts activity. Further investigation is needed to understand how Mats is able to stabilize and/or increase the production of Wts protein.
Mats is a key component of the Hpo signaling pathway
Our previous work has shown that Mats negatively regulates tissue growth by binding to another tumor suppressor Wts and subsequently activating the catalytic activity of Wts kinase (Lai et al, 2005). As loss of mats function leads to tissue overgrowth and tumor development, it suggests that Wts alone is not sufficient to inhibit tissue growth in the absence of Mats. Therefore, Mats is an indispensable component of the Hpo pathway, and Wts activation is dependent not only on Hpo-mediated phosphorylation, but also on Mats binding (Figure 6). Further studies are needed to understand how exactly Wts activation is coordinated by Hpo phosphorylation and Mats binding. In this work, we provide evidence that Mats activation can be mediated by Hpo phosphorylation (Figure 6).
The Hpo signaling pathway plays an important role in growth inhibition and tumor suppression in Drosophila, and this pathway appears to be also critical for tissue growth control and tumor suppression in mammals (Edgar, 2006; Hergovich et al, 2006b). For instance, mammalian NF2 tumor suppressor is a homolog of Drosophila Mer and Ex proteins, which are upstream regulators of the Hpo signaling pathway (Hamaratoglu et al, 2006). Moreover, loss of Lats1 function in mouse causes soft tissue sarcomas and ovarian tumors (St John et al, 1999). Recently, we found that hMats1 can functionally replace fly Mats to suppress tumor development, and Mats1 is mutated in mammalian tumors (Lai et al, 2005). Thus, mechanisms for the control of Hpo signaling might be commonly used across species, and understanding such mechanisms should provide insights into tumor development in mammals. As shown in this report, one mechanism by which Hpo functions to control tissue growth is to target Mats for phosphorylation, and, consequently, Mats is activated to upregulate Wts kinase (Figure 6). Because mammalian Hpo orthologs, Mst kinases, regulates hMats1 in a similar manner, this mechanism is likely used in mammalian cells as well. Therefore, by understanding how Hpo/Mst kinases regulate Mats and Wts/Lats in normal as well as tumor cells, we shall gain valuable insights into tissue growth inhibition and tumor suppression.
Materials and methods
Genetics, immunocytochemistry and microscopy
Fruit flies (Drosophila melanogaster) were cultured under standard conditions. The following strains were used for clonal analysis and overexpression experiments: hpoMGH2 and sav3 (Harvey et al, 2003), hpoBF33, UAS-sav, UAS-hpo, UAS-hpoN and UAS-hpoC (Jia et al, 2003; Udan et al, 2003), UAS-mats, UAS-Myc-wts(6R), FRT82B P[w+]90E, FRT82B matsroo/TM6B and FRT82B matse235/TM6B (Lai et al, 2005), w ey-FLP; FRT82B arm-lacZ/TM6B and w ey-FLP UAS-GFP;Tub-Gal4 FRT82B Tub-Gal80/TM6B (a gift from Jessica Treisman, New York University Medical Center). Mosaic flies that contained loss-of-function mats mutant clones or hpo overexpression clones in third instar larval eye discs were generated using a combination of the Gal4/UAS expression system and the FLP/FRT recombination system (Brand and Perrimon, 1993; Xu and Rubin, 1993; Lee and Luo, 1999). Overexpression of mats, hpo and hpoN during eye development was also achieved by using ey-Gal4 and GMR-Gal4.
For immunostaining of third instar larval eye discs, rabbit anti-β-galactosidase was used to identify mats mutant clones. TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling) assay was used to detect apoptotic cells using an in situ cell death detection kit (Roche, Indianapolis, IN). Alexa Fluor (AF) 488, AF594 and AF680 (Molecular Probes) were used as secondary antibodies. Images were collected with an Olympus Fluoview 300 Confocal Laser Scanning Microscope. GFP signals were directly recorded using a fluorescence microscope. Scanning electron microscopy (SEM) was used to examine adult eye phenotypes.
Plasmid constructs and DNA analysis
Plasmids pAc-hpo-Flag, pAc-hpoKD-Flag and pAc-sav-HA were gifts from Nicolas Tapon, Cancer Research UK London Research Institute, UK (Pantalacci et al, 2003). Plasmids pCMV-HA-mats, pCMV-Myc-wts, pCMV-Myc-wtsKD(K743A) and pGST-mats were previously reported (Lai et al, 2005). Flag-tagged hpo and hpoKD were also cloned in a pCMV vector for protein expression in mammalian cells. A full-length cDNA of hMats1 was cloned into a pGEX vector (Amersham Biosciences Corp, Piscataway, NJ) to generate pGST-hMats1 for making the GST-hMats1 fusion protein in bacteria. Moreover, a full-length cDNA clone of yki (LD21311, which encodes a protein product 366 aa in size) was purchased from the Drosophila Genomics Resource Center (DGRC) and cloned into a pGEX vector to generate pGST-yki for making the GST-Yki fusion protein. Standard methods for DNA cloning and sequencing analysis were used.
Cell culture, protein analysis and kinase assay
Drosophila S2 cells were transiently transfected using Cellfectin (Invitrogen, Carlsbad, CA), maintained in Schneider's medium containing 10% fetal calf serum and collected 36 h after transfection. HEK293T cells were maintained in Dulbecco's modified Eagle's medium containing 10% fetal calf serum and 0.5% penicillin–streptomycin. HEK293T cells were transiently transfected using PolyFect Transfection Reagent (Qiagen, Valencia, CA) and harvested 36 h later. OA treatment of cells was performed by adding 1.0 μM OA (Sigma, St Louis, MO) to the culture medium for 30 min at 37°C unless otherwise indicated. All lysis, co-immunoprecipitation and Western blots were performed as described previously (Lai et al, 2005). Recombinant full-length rat Mst2 protein (rMst2) was purchased from Upstate (Charlottesville, VA). The following antibodies were used as primary antibodies: rabbit polyclonal antibodies against Myc epitope and GFP (Santa Cruz Biotechnology Inc., Santa Cruz, CA), M2 mouse monoclonal antibody against Flag (Sigma), 12CA5 mouse monoclonal antibody against HA (Roche) and rabbit polyclonal antibody against hMst1 (Upstate). Our anti-Mats antibody cross-reacted with human and mouse Mats proteins. Horseradish peroxidase- (HRP-) conjugated antibodies against mouse IgG and rabbit IgG (Amersham) were used as secondary antibodies for Western blot analysis.
For TAP and protein staining, HA-mats was cloned into the pNTAP vector in-frame with the SBP and CBP affinity tags located at the 5′ end of the fusion gene. Following the manufacturer's protocol, protein extracts derived from transfected HEK293T cells were subjected to two consecutive purification steps using InterPlay Mammalian TAP system (Stratagene, La Jolla, CA). Molecular Probes Pro-Q® Diamond phosphoprotein gel stain was used to selectively stain phosphoproteins in polyacrylamide gels in conjunction with SYPRO® Ruby protein gel stain (Bio-Rad Laboratories Inc., Hercules, CA) to measure total proteins. The gel was imaged and documented using Molecular Imager FX (Bio-Rad).
For in vitro kinase assays, Myc-Wts was immunoprecipitated and tested for its kinase activity in kinase buffer (50 mM Tris (pH 7.4), 60 mM potassium acetate, 10 mM MgCl2, 1 mM DTT, 10 μM ATP) and 10 μCi of [γ-32P]ATP at room temperature for 30 min. Hpo-Flag or HpoKD-Flag immunoprecipitates were washed and resuspended in Hpo kinase buffer (20 mM HEPES (pH 7.6), 20 mM MgCl2, 5 mM NaF, 1 mM sodium orthovanadate, 20 mM DDT, 20 μM ATP, 20 mM β-glycerophosphate) and 10 μCi of [γ-32P]ATP and 2 μg of GST or GST fusion proteins where indicated. Reactions were incubated for 20 min at 30°C and stopped by the addition of 6 × SDS–PAGE sample buffer and boiling, and subsequently analyzed by electrophoresis and autoradiography. For pretreatment of Mats by Hpo, soluble GST-Mats or GST-hMats1 was phosphorylated by immobilized Hpo-Flag or HpoKD-Flag in the presence of non-radioactive ATP. After the cold phosphorylation, GST-Mats or GST-hMats1 treated with Hpo or HpoKD was carefully collected as supernatant. Subsequently, GST-Mats or GST-hMats1 was added to immobilized Wts kinase precipitates for phosphorylation reaction in the presence of radioactive [γ-32P]ATP.
Acknowledgments
We thank Douglas Cavener for his encouragement in this work and helpful comments on an initial version of this manuscript, Xuqing Zhang for making the pGST-yki construct, and Georg Halder, Iswar Hariharan, Jin Jiang, Nicolas Tapon, Jessica Treisman and the Bloomington Drosophila Stock Center and Drosophila Genomics Resource Center for reagents and fly strains. This work was supported by a grant to Z-CL from the National Science Foundation (IBN-0348262).
References
- Bichsel SJ, Tamaskovic R, Stegert MR, Hemmings BA (2004) Mechanism of activation of NDR (nuclear Dbf2-related) protein kinase by the hMOB1 protein. J Biol Chem 279: 35228–35235 [DOI] [PubMed] [Google Scholar]
- Bothos J, Tuttle RL, Ottey M, Luca FC, Halazonetis TD (2005) Human LATS1 is a mitotic exit network kinase. Cancer Res 65: 6568–6575 [DOI] [PubMed] [Google Scholar]
- Brand A, Perrimon N (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415 [DOI] [PubMed] [Google Scholar]
- Chan EH, Nousiainen M, Chalamalasetty RB, Schafer A, Nigg EA, Sillje HH (2005) The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene 24: 2076–2086 [DOI] [PubMed] [Google Scholar]
- Edgar BA (2006) From cell structure to transcription: Hippo forges a new path. Cell 124: 267–273 [DOI] [PubMed] [Google Scholar]
- Emoto K, He Y, Ye B, Grueber WB, Adler PN, Jan LY, Jan YN (2004) Control of dendritic branching and tiling by the Tricornered-kinase/Furry signaling pathway in Drosophila sensory neurons. Cell 119: 245–256 [DOI] [PubMed] [Google Scholar]
- Hamaratoglu F, Willecke M, Kango-Singh M, Nolo R, Hyun E, Tao C, Jafar-Nejad H, Halder G (2006) The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat Cell Biol 8: 27–36 [DOI] [PubMed] [Google Scholar]
- Hariharan IK (2006) Growth regulation: a beginning for the hippo pathway. Curr Biol 16: R1037–1039 [DOI] [PubMed] [Google Scholar]
- Hariharan IK, Bilder D (2006) Regulation of imaginal disc growth by tumor-suppressor genes in Drosophila. Annu Rev Genet 40: 335–361 [DOI] [PubMed] [Google Scholar]
- Harvey KF, Pfleger CM, Hariharan IK (2003) The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell 114: 457–467 [DOI] [PubMed] [Google Scholar]
- He Y, Emoto K, Fang X, Ren N, Tian X, Jan YN, Adler PN (2005a) Drosophila Mob family proteins interact with the related tricornered (Trc) and warts (Wts) kinases. Mol Biol Cell 16: 4139–4152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Fang X, Emoto K, Jan YN, Adler PN (2005b) The tricornered Ser/Thr protein kinase is regulated by phosphorylation and interacts with furry during Drosophila wing hair development. Mol Biol Cell 16: 689–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hergovich A, Bichsel SJ, Hemmings BA (2005) Human NDR kinases are rapidly activated by MOB proteins through recruitment to the plasma membrane and phosphorylation. Mol Cell Biol 25: 8259–8272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hergovich A, Schmitz D, Hemmings BA (2006a) The human tumour suppressor LATS1 is activated by human MOB1 at the membrane. Biochem Biophys Res Commun 345: 50–58 [DOI] [PubMed] [Google Scholar]
- Hergovich A, Stegert MR, Schmitz D, Hemmings BA (2006b) NDR kinases regulate essential cell processes from yeast to humans. Nat Rev Mol Cell Biol 7: 253–264 [DOI] [PubMed] [Google Scholar]
- Hipfner DR, Cohen SM (2004) Connecting proliferation and apoptosis in development and disease. Nat Rev Mol Cell Biol 5: 805–815 [DOI] [PubMed] [Google Scholar]
- Hou MC, Guertin DA, McCollum D (2004) Initiation of cytokinesis is controlled through multiple modes of regulation of the Sid2p–Mob1p kinase complex. Mol Cell Biol 24: 3262–3276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou MC, Salek J, McCollum D (2000) Mob1p interacts with the Sid2p kinase and is required for cytokinesis in fission yeast. Curr Biol 10: 619–622 [DOI] [PubMed] [Google Scholar]
- Huang J, Wu S, Barrera J, Matthews K, Pan D (2005) The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell 122: 421–434 [DOI] [PubMed] [Google Scholar]
- Jia J, Zhang W, Wang B, Trinko R, Jiang J (2003) The Drosophila Ste20 family kinase dMST functions as a tumor suppressor by restricting cell proliferation and promoting apoptosis. Genes Dev 17: 2514–2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice RW, Zilian O, Woods DF, Noll M, Bryant PJ (1995) The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Gene Dev 9: 534–546 [DOI] [PubMed] [Google Scholar]
- Kango-Singh M, Nolo R, Tao C, Verstreken P, Hiesinger PR, Bellen HJ, Halder G (2002) Shar-pei mediates cell proliferation arrest during imaginal disc growth in Drosophila. Development 129: 5719–5730 [DOI] [PubMed] [Google Scholar]
- Komarnitsky SI, Chiang YC, Luca FC, Chen J, Toyn JH, Winey M, Johnston LH, Denis CL (1998) DBF2 protein kinase binds to and acts through the cell cycle-regulated MOB1 protein. Mol Cell Biol 18: 2100–2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Z-C, Wei X, Shimizu T, Ramos E, Rohrbaugh M, Nikolaidis N, Ho LL, Li Y (2005) Control of cell proliferation and apoptosis by mob as tumor suppressor Mats. Cell 120: 675–685 [DOI] [PubMed] [Google Scholar]
- Lee KK, Yonehara S (2002) Phosphorylation and dimerization regulate nucleocytoplasmic shuttling of mammalian STE20-like kinase (MST). J Biol Chem 277: 12351–12358 [DOI] [PubMed] [Google Scholar]
- Lee SE, Frenz LM, Wells NJ, Johnson AL, Johnston LH (2001) Order of function of the budding-yeast mitotic exit-network proteins Tem1, Cdc15, Mob1, Dbf2, and Cdc5. Curr Biol 11: 784–788 [DOI] [PubMed] [Google Scholar]
- Lee T, Luo L (1999) Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron 22: 451–461 [DOI] [PubMed] [Google Scholar]
- Mah AS, Jang J, Deshaies RJ (2001) Protein kinase Cdc15 activates the Dbf2–Mob1 kinase complex. Proc Natl Acad Sci USA 98: 7325–7330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno CS, Lane WS, Pallas DC (2001) A mammalian homolog of yeast MOB1 is both a member and a putative substrate of striatin family-protein phosphatase 2A complexes. J Biol Chem 276: 24253–24260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolo R, Morrison CM, Tao C, Zhang X, Halder G (2006) The bantam microRNA is a target of the hippo tumor-suppressor pathway. Curr Biol 16: 1895–1904 [DOI] [PubMed] [Google Scholar]
- O'Neill E, Rushworth L, Baccarini M, Kolch W (2004) Role of the kinase MST2 in suppression of apoptosis by the proto-oncogene product Raf-1. Science 306: 2267–2270 [DOI] [PubMed] [Google Scholar]
- Pantalacci S, Tapon N, Leopold P (2003) The Salvador partner Hippo promotes apoptosis and cell-cycle exit in Drosophila. Nat Cell Biol 5: 921–927 [DOI] [PubMed] [Google Scholar]
- Stavridi ES, Harris KG, Huyen Y, Bothos J, Verwoerd PM, Stayrook SE, Pavletich NP, Jeffrey PD, Luca FC (2003) Crystal structure of a human Mob1 protein: toward understanding Mob-regulated cell cycle pathways. Structure 11: 1163–1170 [DOI] [PubMed] [Google Scholar]
- St John MA, Tao W, Fei X, Fukumoto R, Carcangiu ML, Brownstein DG, Parlow AF, McGrath J, Xu T (1999) Mice deficient of Lats1 develop soft-tissue sarcomas, ovarian tumours and pituitary dysfunction. Nat Genet 21: 182–186 [DOI] [PubMed] [Google Scholar]
- Tapon N, Harvey KF, Bell DW, Wahrer DCR, Schiripo TA, Haber DA, Hariharan IK (2002) salvador promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell 110: 467–478 [DOI] [PubMed] [Google Scholar]
- Thompson BJ, Cohen SM (2006) The Hippo pathway regulates the bantam microRNA to control cell proliferation and apoptosis in Drosophila. Cell 126: 767–774 [DOI] [PubMed] [Google Scholar]
- Udan RS, Kango-Singh M, Nolo R, Tao C, Halder G (2003) Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat Cell Biol 5: 914–920 [DOI] [PubMed] [Google Scholar]
- Wu S, Huang J, Dong J, Pan D (2003) hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell 114: 445–456 [DOI] [PubMed] [Google Scholar]
- Xu T, Rubin GM (1993) Analysis of genetic mosaics in developing and adult Drosophila tissues. Development 117: 1223–1237 [DOI] [PubMed] [Google Scholar]
- Xu T, Wang W, Zhang S, Stewart RA, Yu W (1995) Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes putative protein kinase. Development 121: 1053–1063 [DOI] [PubMed] [Google Scholar]
- Yang X, Yu K, Hao Y, Li DM, Stewart R, Insogna KL, Xu T (2004) LATS1 tumour suppressor affects cytokinesis by inhibiting LIMK1. Nat Cell Biol 6: 609–617 [DOI] [PubMed] [Google Scholar]


