Abstract
Uric acid, the naturally occurring product of purine metabolism, is a strong peroxynitrite scavenger, as demonstrated by the capacity to bind peroxynitrite but not nitric oxide (NO) produced by lipopolysaccharide-stimulated cells of a mouse monocyte line. In this study, we used uric acid to treat experimental allergic encephalomyelitis (EAE) in the PLSJL strain of mice, which develop a chronic form of the disease with remissions and exacerbations. Uric acid administration was found to have strong therapeutic effects in a dose-dependent fashion. A regimen of four daily doses of 500 mg/kg uric acid was required to promote long-term survival regardless of whether treatment was initiated before or after the clinical symptoms of EAE had appeared. The requirement for multiple doses is likely to be caused by the rapid clearance of uric acid in mice which, unlike humans, metabolize uric acid a step further to allantoin. Uric acid treatment also was found to diminish clinical signs of a disease resembling EAE in interferon-γ receptor knockout mice. A possible association between multiple sclerosis (MS), the disease on which EAE is modeled, and uric acid is supported by the finding that patients with MS have significantly lower levels of serum uric acid than controls. In addition, statistical evaluation of more than 20 million patient records for the incidence of MS and gout (hyperuricemic) revealed that the two diseases are almost mutually exclusive, raising the possibility that hyperuricemia may protect against MS.
The induction of the nitric oxide synthase isoform (NOS-2) in the central nervous system (CNS) commonly associated with cells of the macrophage/monocyte lineage is a characteristic feature of experimental allergic encephalomyelitis (EAE) (see refs. 1–7 for examples). Moreover, production of the free radical nitric oxide (NO) in CNS tissue of mice has been correlated with the development of clinical signs of the disease (2, 7). The contribution of NO in the etiology of EAE has been confirmed in mice that were immunized with proteolipid protein peptide but failed to develop EAE when treated with a compound that inactivates NO (8).
It remains unproven whether NO, which has a short half-life in vivo, exerts a toxic effect on CNS cells directly or through the formation of a more toxic compound, the production of which is directly related to the induction of elevated NO levels in brain tissue. Peroxynitrite (ONOO−), a potent oxidant that is formed by the rapid combination of NO with superoxide (O2−), can be formed in an inflammatory response (9) and can cause a variety of toxic effects, including lipid peroxidation (10) and tyrosine nitration (11, 12). It therefore has been suggested that peroxynitrite is responsible for a significant proportion of the inflammatory damage attributed to NO (12). We have shown that treatment with uric acid, a naturally occurring compound that selectively binds and inactivates peroxynitrite (13), inhibits the onset of clinical disease in an acute, aggressive form of mouse EAE, a result expected only if peroxynitrite is the more toxic molecule (8). In the present investigation, we have extended our studies of the therapeutic effect of uric acid in EAE, with an emphasis on the treatment of mice already showing clinical signs of disease and on long-term survival. As interferon-γ (IFN-γ) is a major contributor to the induction of iNOS (14), the fact that inhibition of IFN-γ with mAbs (15) or targeted disruption of the IFN-γ gene can enhance susceptibility to EAE (16) has raised questions concerning the role of NO-dependent molecules in the pathogenesis of this disease. We therefore have assessed the effects of treatment with uric acid on EAE in mice lacking IFN-γ receptors. We have also examined the possible relationship between uric acid levels and multiple sclerosis (MS), because nitrotyrosine residues on proteins, which are stable markers of peroxynitrite-mediated damage, have been shown to be associated with plaque areas in brain of MS patients (8), suggesting that, as in EAE, peroxynitrite formation may be involved in the pathogenesis of MS. We therefore compared serum uric acid levels in MS patients and controls and surveyed gout (hyperuricemic) patients for their incidence of MS.
MATERIALS AND METHODS
In Vitro Assessment of the Effect of Uric Acid on NO and Peroxynitrite Formation.
RAW 264.7 cells, obtained from the American Type Culture Collection, were cultured on 24-well plates in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 50 units of penicillin, 50 μg/ml of streptomycin, and 5 mM l-glutamine. When cells reached 80% confluence (106 per well), they were activated with 1 μg/ml of lipopolysaccharide (LPS) (Escherichia coli O55:B5). For measurement of NO formation, cells were activated in the absence or the presence of various concentrations of uric acid and n-methyl-l-arginine (l-NMMA; Sigma) for 24 hr. Nitrite was measured in culture media by using a technique based on the Griess reaction (17). To assess peroxynitrite in the cultures, cells were washed twice with PBS after 24-hr incubation with LPS cells, and 1 ml of PBS containing 5 μM dihydrorhodamine 123 (DHR123), and the indicated concentrations of uric acid or l-NMMA were added. As peroxynitrite specificity controls superoxide dismutase (Sigma) and catalase (Sigma) were added to replicate cultures at 100 μg/ml. After an additional 1-hr incubation at 37°C, rhodamine 123 luminescence was measured in a fluorimeter with an excitation wavelength of 485 nm and a detection wavelength of 530 nm. Catalase, which breaks down hydrogen peroxide, had no effect on DHR123 oxidation, whereas superoxide dismutase completely blocked DHR123 oxidation (data not shown). To assess the effects of uric acid on peroxynitrite-mediated toxicity, RAW cells at 80% confluence were washed twice in medium without fetal bovine serum and phenol red, and the indicated concentrations of the peroxynitrite donor SIN-1 (Alexis Biochemicals, San Diego, CA) were added with and without 1 mM uric acid. After 24-hr incubation at 37°C toxicity was measured by propidium iodide (Sigma) staining (18).
Induction of EAE.
EAE was induced in 8- to 9-week-old female PLSJL mice (The Jackson Laboratory) and in IFN Gamma Ro/o 129/Sv mice (IFN-γR KO, a kind gift of Michel Aguet, The Swiss Institute for Experimental Cancer Research, Lausanne), which were generated by targeted gene disruption as described elsewhere (19), by subcutaneous immunization with 100 μg of myelin basic protein (MBP) in complete Freund’s adjuvant (CFA) on day 0, followed by i.p. injection of 400 ng pertussis toxin (Sigma, catalog no. P-0317) on days 0 and 2. MBP was prepared in the laboratory by using an established technique (20). In this model, EAE can range from acute onset, leading to death in over 80% of the mice by 20 days after immunization, to a less severe form of the disease, with initial symptoms appearing in only 60–80% of the mice from 15 to 30 days after immunization. Relapsing disease is common in survivors of the initial course of the disease. In the IFN-γR KO mice the disease is acute and severe with approximately 80% mortality. Clinical severity of EAE was assessed a minimum of twice daily by at least two independent investigators. Scores were assigned on the basis of the presence of the following symptoms: 0, normal mouse; 1, piloerection, tail weakness; 2, tail paralysis; 3, tail paralysis plus hindlimb weakness; 4, tail paralysis plus partial hindlimb paralysis; 5, total hindlimb paralysis; 6, hind and forelimb paralysis; 7, moribund/dead.
Treatment Regimen and Determination of Serum Uric Acid Levels.
Uric acid, obtained from Sigma, (catalog no. U-0881) was used as a suspension in 0.8% saline. Mice received either 500 mg/kg of uric acid in a 100-μl saline suspension per injection or saline alone as a control. Treatment schedules within individual groups of mice are detailed in the figure legends. Serum uric acid levels were measured by using a quantitative enzymatic assay (Sigma, catalog no. 685–10) according to the manufacturer’s protocol (Sigma, procedure no. 685), and the results were standardized by using a commercial uric acid standard solution (Sigma, catalog no. 685–1). Comparison of serum uric acid levels in MS patients and controls was performed in collaboration with Inglis House, a palliative care institution in Philadelphia. Subjects were age- and sex-matched MS and control patients, the latter predominantly with spinal cord injury or Parkinson’s disease. The 46 MS patients (diagnosis confirmed by MRI) as well as the control group contained equal numbers of males and females. To control for the significant influence of diet on serum uric acid levels, the institutionalized subjects all received the same diet for 5 days before collection of serum for assessment of uric acid levels, and blood samples were obtained from all subjects before breakfast. Acetylsalicylic acid, thiazide diuretics, and certain other drugs have been reported to modulate serum uric acid levels (e.g., refs. 21 and 22). Individuals treated with such drugs therefore were excluded from this study. Uric acid levels were determined by the clinical laboratories at Thomas Jefferson University Hospital by using a commercially available enzymatic, colorimetric assay according to the manufacturer’s instructions (Boehringer Mannheim document 011389402–1092). Statistical analysis was performed by using the systat program. Significance was calculated by the Wilcoxon rank sum test.
Survey of the Incidence of MS and Gout.
Outpatient records from the Health Care Financing Administrations 1995 database were searched for individuals with MS (ICD-9 code 340) and gout (ICD-9 code 2740).
RESULTS
Effects of Uric Acid on Peroxynitrite and NO in Vitro.
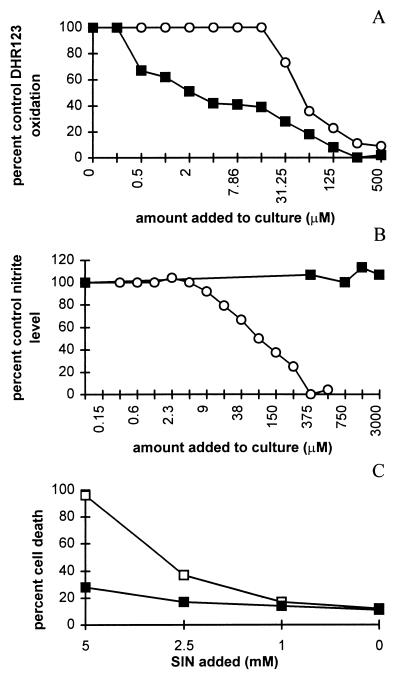
To confirm that uric acid scavenges peroxynitrite without affecting the mechanisms involved in the production of the molecule, we examined the effects of uric acid on NO production and peroxynitrite accumulation in vitro. Preincubation of LPS-stimulated RAW cells with uric acid for 1 hr inhibited the oxidation of DHR123 mediated by peroxynitrite (Fig. 1A) but did not significantly diminish NO production as measured by the accumulation of nitrate and nitrite during 24 hr of culture (Fig. 1B). l-NMMA, an inhibitor of NOS, suppressed both NO production and DHR123 oxidation. Because DHR123 oxidation in this assay also was inhibited by superoxide dismutase, but not by catalase (data not shown), we conclude that peroxynitrite is the likely mediator of the DHR123 oxidation. Exogenous addition of the peroxynitrite donor SIN-1 to RAW cells caused cell death that was inhibited by the addition of uric acid (Fig. 1C).
Figure 1.
In vitro effects of uric acid on NO production and peroxynitrite detection in cultures of RAW cells stimulated by LPS. RAW 264.7 cells were activated by LPS in the presence of various concentrations of uric acid (▪) or l-NMMA (○), and peroxynitrite (A) and NO formation (B) were assessed as described in Materials and Methods. To assess peroxynitrite, cells were washed twice with PBS after 24-hr incubation with LPS cells and 1 ml of PBS containing 5 μM DHR123 and the indicated concentrations of uric acid or l-NMMA were added. After an additional 1-hr incubation at 37°C, rhodamine fluorescence was measured as an indicator of peroxynitrite production. For measurement of NO formation, cells were stimulated for 24 hr in the presence of uric acid, and nitrite was measured in the culture medium. Data are presented as percentage of peroxynitrite or nitrite detected in replicate cultures without added uric acid or l-NMMA. (C) The peroxynitrite donor SIN-1 was titrated into RAW cells cultured in the absence (□) or presence of 1 mM uric acid (▪). Twenty-four hours later the percentage cell death was calculated by propidium iodide staining as described in Materials and Methods.
Uric Acid Treatment of Mice with Pre-Existing EAE.
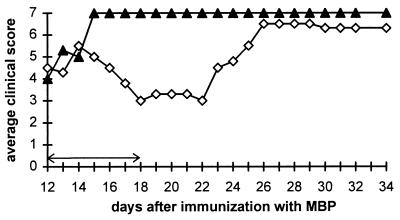
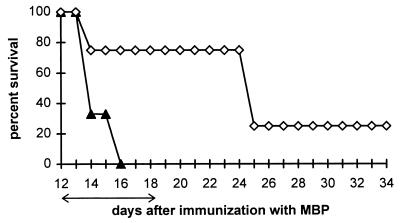
Our previous experiments demonstrated that two daily doses of 10 mg of uric acid, initiated 5 days after immunization with MBP or proteolipid peptide, delays the onset of EAE (8). To determine whether uric acid is also effective in the treatment of mice with clinical signs of EAE, PLSJL mice were immunized with MBP and left untreated until severe disease developed in the animals at day 12. Mice then were divided into two groups, with and without symptoms, and half of the mice in each group were treated with uric acid for 7 days. As shown in Fig. 2, the mice receiving uric acid continued to deteriorate for 2 days after the start of treatment, but then showed a dramatic improvement in clinical signs of the disease. When treatment was discontinued 18 days after immunization, the clinical status of the mice remained stable for 4 days but then rapidly deteriorated. Nevertheless, 80% of the treated animals survived to 24 days after immunization with MBP as opposed to none of the untreated controls (Fig. 3). During the period of treatment, mice that had not yet developed clinical EAE at the initiation of treatment developed less severe disease than controls (average clinical score at day 21 postimmunization 0.5 vs. 3.8). One-third of the untreated animals died during the course of the treatment or shortly thereafter, whereas none of the uric acid-treated mice died (results not shown). In mice that were healthy and in mice with signs of EAE at the start of treatment, the therapeutic benefit of uric acid ceased approximately 4 days after termination of its administration. From these experiments, we concluded that uric acid is effective in the treatment of EAE both before and after the development of the clinical disease, but that the therapeutic effect is dependent on the continued administration of uric acid.
Figure 2.
Effect of uric acid treatment on clinical signs of pre-existing EAE in PLSJL mice. Groups of 10 female PLSJL mice were immunized with MBP in CFA. Twelve days later, mice with clinical signs either were treated with uric acid (◊) twice daily with 10 mg per dose for 7 days (arrows) or left untreated (▴). Clinical score was assessed twice daily as described in Materials and Methods and is presented as an average.
Figure 3.
Effect of uric acid treatment on survival of PLSJL mice with pre-existing EAE. Groups of 10 female PLSJL mice were immunized with MBP in CFA. Twelve days later, mice with clinical signs either were treated with uric acid (◊) twice daily at 10 mg per dose for 7 days (arrows) or left untreated (▴).
Effect of Continued Administration of 10 mg of Uric Acid Twice Daily in EAE.
The experiments above clearly show that two doses of 10 mg of uric acid are therapeutic in EAE but that symptoms of the disease either develop or return when the treatment is discontinued. In experiments to determine whether prolonged treatment with the same dose regimen of uric acid prevents EAE over extended periods of time, two daily doses of 500 mg/kg of uric acid were found to delay the onset of clinical EAE, but the mice eventually developed severe disease (Fig. 4). Although uric acid treatment prolonged the survival of MBP-immunized PLSJL mice, long-term survival rates of the treated and control groups of mice did not differ significantly (data not shown).
Figure 4.
Effect of continued administration of uric acid on the onset of clinical signs of acute EAE in PLSJL mice. Groups of 10 female PLSJL mice were immunized with MBP in CFA as described in Materials and Methods and treated twice daily starting 5 days later with saline (▴) or 10 mg of uric acid (◊). Clinical score was assessed twice daily and is presented as an average.
Serum Uric Acid Levels in Mice after Treatment with Uric Acid.
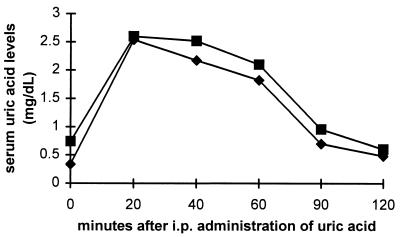
In contrast to humans, in which uric acid is the end product of purine metabolism and present in serum at significant concentrations, mice metabolize uric acid a step further to allantoin, which does not have peroxynitrite scavenging activity (13) nor any noticeable effect on EAE (data not shown). Thus, to determine whether the ultimate failure of two daily doses of 500 mg/kg of uric acid to prevent EAE is the result of rapid clearance of the molecule such that therapeutic levels are maintained only transiently, we measured serum uric acid levels in mice after the administration of a single dose of 10 mg of uric acid; the elevated serum uric acid levels seen by 20 min after its administration virtually disappear within 2 hr (Fig. 5).
Figure 5.
Serum uric acid levels after single i.p. administration of uric acid. Groups of 5–10 BALB/c (▪) and SWX/J14 (⧫) mice were given a single i.p. dose of 10 mg of uric acid and bled at the indicated intervals. Uric acid levels in sera obtained before and after treatment are presented as a mean for each time point.
Effect of Increasing Uric Acid Dose Frequency on Development of and Recovery from EAE.
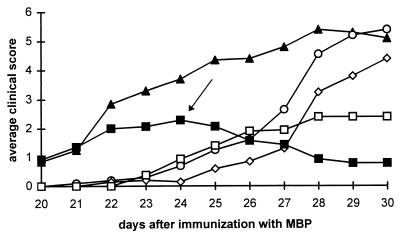
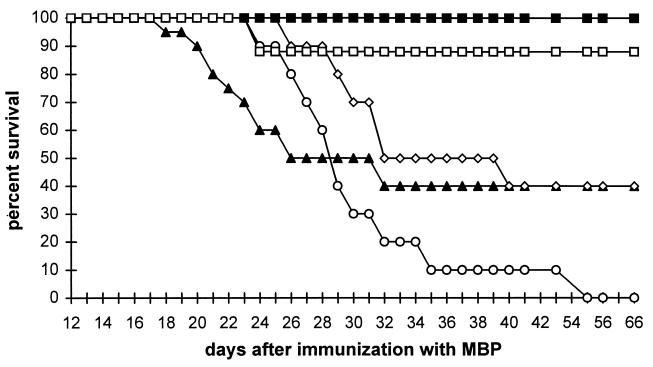
If the rapid clearance of uric acid is responsible for the eventual development of EAE in mice treated twice daily, rather than the development of a second peroxynitrite-independent pathogenic mechanism, a more comprehensive uric acid dose regimen would be expected to have a greater therapeutic effect. Comparison of the effects of three uric acid administration schedules, ranging from one to four daily doses of 500 mg/kg each, on the development of EAE (Fig. 6) indicated that all three dose regimens caused a marked delay in the appearance of EAE clinical symptoms, but the best clinical outcome in the long term was achieved with four daily doses of uric acid. In addition, four daily doses of 500 mg/kg of uric acid reversed disease progression in a group of MBP-immunized but otherwise untreated mice that developed EAE but survived until 24 days after injection (Fig. 6). The long-term survival rate of PLSJL mice after immunization with MBP was the highest in animals receiving four daily doses of uric acid (Fig. 7). Most of these animals survived regardless of whether treatment, which continued until day 35 after MBP administration, started when the mice were still healthy (day 5) or sick (day 24). In the experiment shown in Figs. 6 and 7, 14 of 15 mice treated with four daily doses of uric acid survived until 35 days after MBP immunization and when uric acid administration was discontinued, were alive a month later without additional treatment. Whether or not mice received uric acid, those surviving the first month after immunization showed signs of relapsing EAE (average clinical score of 5), from which they spontaneously recovered.
Figure 6.
Comparison of the protective effect of one, two, and four daily doses of uric acid against EAE. Groups of 10 PLSJL mice were immunized with MBP as described in Materials and Methods. Starting 5 days later and continuing for 30 days, groups of 10 mice were treated with 10 mg of uric acid i.p. once (○), twice (◊), or four (□) times daily or with saline (▴). One group of 7/10 mice surviving without treatment to 24 days after immunization received four doses per day from day 24 (arrow) through day 35 (▪); the three mice that died before the start of treatment are excluded from the graph. Results are presented as an average of the clinical score.
Figure 7.
Survival of PLSJL mice immunized with MBP in CFA and treated with 10 mg of uric acid once, twice, or four times daily starting at 5 or 24 days after immunization. Shown are the survival curves of the mice described in the legend to Fig. 6. Starting 5 days after immunization with MBP and continuing for 30 days, the groups of 10 mice were treated with 10 mg of uric acid i.p. once (○), twice (◊), or four (□) times daily or with saline (▴). A group of seven mice surviving without treatment to 24 days after immunization received four doses per day starting at that point (▪) through day 35 after immunization.
Effect of Uric Acid Treatment on EAE in IFN-γR KO Mice.
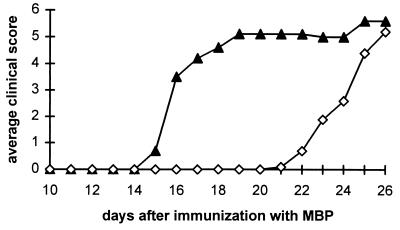
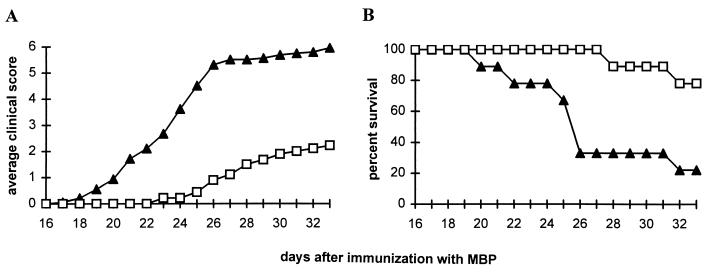
Inhibition of the expression or activity of IFN-γ increases susceptibility to the induction of EAE (15, 16). As shown in Fig. 8, EAE also can be readily triggered in IFN-γR KO mice. Immunization of IFN-γR KO with MBP elicits a progressive, severe disease with clinical manifestations resembling those of EAE. Four daily doses of 500 mg/kg uric acid, beginning 19 days after immunization, delayed the appearance and reduced the severity of clinical signs of EAE (Fig. 8A) as well as prolonged the survival of the mice (Fig. 8B).
Figure 8.
Effect of uric acid treatment on the clinical signs of EAE in IFN-γR KO mice. Groups of 10 female and eight male IFN-γR KO mice were immunized with MBP in CFA. Nineteen days later, half of the mice in each group began four times daily i.p. treatment with either uric acid (□), 10 mg per dose, or saline (▴). Clinical score was assessed twice daily as described in Materials and Methods, and is presented as an average in A. The percent survival of the treated and control mice is shown in B.
Uric Acid and MS.
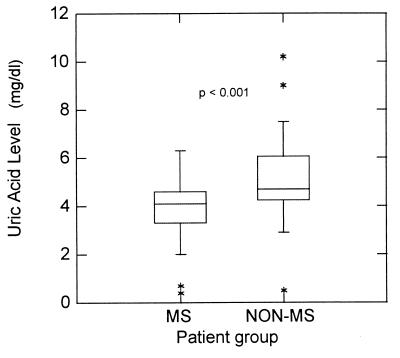
Unlike mice, humans have relatively high levels of serum uric acid (4–6 mg/dl) (23). If our studies with EAE bear significance to MS, any contribution of peroxynitrite to the pathogenesis of MS might be reflected in altered serum uric acid levels. Thus, we conducted two clinically oriented studies to determine: (i) whether serum uric acid levels differ in MS patients versus controls; and (ii) whether hyperuricemia may preclude the development of MS. In the first study, 46 age- and sex- matched MS and other primarily neurological inpatients were placed on the same diet for 5 days to prevent contributions to uric acid levels from varied diets, and their serum uric acid levels were determined. MS patients were found to have significantly lower serum uric acid levels than those of the controls (P < 0.001) (Fig. 9). In the second study, the records of 20,212,505 outpatients enrolled in Medicare and Medicaid in 1995 were surveyed for patients with MS, gout, and both conditions together. As shown in Table 1, although the distributions of MS and gout in this population should lead to approximately 62 individuals with both diseases, only four such individuals were identified.
Figure 9.
Distribution of serum uric acid levels in MS and non-MS patients. Sera were collected and uric acid levels were determined as described in Materials and Methods. For each group of 46 subjects, the majority of the values obtained fell in the range of the boxes plus bars, with outlying values denoted by ∗. The central 50% of the values fell within the boxes that also contain the median line for the group. P < 0.001 by the Wilcoxon rank sum test.
Table 1.
Survey of the incidence of MS and gout
| Patients | Number | Probability |
|---|---|---|
| Total | 20,212,505 | |
| Gout | 36,733 | 0.0018 |
| MS | 34,607 | 0.0017 |
| Both gout and MS (predicted) | 62 | 0.0000031 |
| Both gout and MS (actual) | 4 | 0.0000002 |
DISCUSSION
A variety of investigations have demonstrated that EAE can be suppressed by inhibiting inducible NO synthase induction (iNOS) (3, 8, 24, 25) or scavenging NO (8), suggesting that NO, either as an effector molecule or precursor, is involved in the pathogenesis of EAE. Our previous (8) and present results, identify peroxynitrite as a major factor in the pathogenesis of EAE and possibly MS, consistent with the association of the two diseases with iNOS induction in the CNS, the detection of nitrotyrosine residues on cell debris in the plaque areas of MS brain, and with the efficacy of uric acid, a known peroxynitrite scavenger, in treating EAE.
Peroxynitrite may exert its toxic effect through at least two mechanisms. The first is through nitration of macromolecules such as tyrosine or possibly cysteine, which can be catalyzed by superoxide dismutase (12). For example, in amyotrophic lateral sclerosis, nitration of tyrosine in the neuronal filaments of motor neurons leads to neuronal dysfunction (26). In MS, we have detected nitrotyrosine accumulation in plaque areas (8), and antibodies reacting with nitrocysteine have been detected in the sera of MS patients (27). Moreover mitochondrial respiration in cultured neurons has been shown to be damaged by peroxynitrite (28).
The second peroxynitrite-mediated toxic mechanism is through its action as a “selective” oxidant (29). There is still controversy whether peroxynitrite forms, by homeolytic cleavage, the highly reactive hydroxyl radical (5). Nevertheless, accumulating evidence indicates that the peroxidation of lipids, which has been observed in MS (30), and, more importantly, the oxidation of deoxyguanine to various species including 8-oxodeoxyguanosine, which may lead to DNA strand breakage or subsequent mutations in DNA (31–34), play significant roles in peroxynitrite-mediated toxicity. Although all of these peroxynitrite-mediated toxic effects may contribute to cell death, DNA strand breakage is the obligatory stimulus for the induction of the nuclear enzyme poly-ADP ribosyl synthetase (PARS). PARS activation also has been implicated in pathogenesis, possibly causing cell death through energy depletion and the induction of apoptosis. In this regard, it should be noted that apoptotic death of cells has been detected in MS (35), and recent studies have implicated Fas and Fas ligand-mediated apoptosis in the pathogenesis of EAE (36, 37). In both MS and EAE, it is possible that the end result of peroxynitrite production in the CNS is the induction of apoptosis in resident cells. In addition to MS and amyotrophic lateral sclerosis, Alzheimer’s disease also might be associated with the contribution of peroxynitrite formation (38, 39). Our studies with the known peroxynitrite scavenger uric acid attest to the importance of peroxynitrite in the pathogenesis of EAE. Uric acid treatment had significant therapeutic value in EAE even after the onset of clinical symptoms of the disease. It is also clear that the clinical signs of EAE appeared or progressed when treatment was discontinued, which is expected if uric acid is scavenging a toxic product rather than interfering with the induction of the pathogenic response. With each injection, uric acid levels were only transiently elevated in the serum of treated mice so that administration four times daily was the minimum required to promote recovery and long-term survival of mice with EAE.
The induction of iNOS in response to inflammatory stimuli, and resulting NO production, is compromised in IFN-γR KO mice (40). Nevertheless, clinical signs of EAE in these mice can be inhibited by treatment with uric acid. Although the pathway responsible for the production of the NO required to form peroxynitrite in this model remains to be determined, this finding serves to emphasize the importance of peroxynitrite as a neuropathogenic molecule in EAE.
In the case of MS, we observed significantly lower levels of uric acid in blood of these patients as compared with patients with other neurological diseases. It is thus possible that inadequate protection against the activity of peroxynitrite by its scavenger causes progressive damage to the CNS in MS patients. The decrease in uric acid levels also may be attributed to its oxidation by peroxynitrite and formation of allantoin, described to occur in humans (41). Additional studies to assess allantoin levels in the sera of MS versus control patients should help to answer this question. In either case, it appears that high serum uric acid levels protect against the development of MS because gout and MS are virtually self-exclusive. These results raise the possibility that the natural biological product, uric acid, or a more soluble peroxynitrite scavenger that penetrates the blood brain-barrier more readily than uric acid, might have clinical utility in the treatment of MS.
Acknowledgments
We thank Drs. David Romanoff and Paul Goldberg and the personnel and patients of Inglis House, Philadelphia, for their participation. We thank Tanya Mikheeva for excellent technical help, Dr. Jack W. Snyder II of Environmental Medicine and Toxicology, Thomas Jefferson University Hospital, for arranging the uric acid level determinations, and Dr. Walter Hauck, Department of Clinical Pharmacology, Thomas Jefferson University Hospital for performing the statistical analyses. We thank Dr. Michel Aguet, Swiss Institute for Experimental Cancer Research for providing breeding pairs of IFN-γR KO mice. We also thank Judith A. Giles of the Division of Data Liaison and Distribution, Enterprise Database Group, Office of Information Services, Health Care Financing Administration and Dr. Frank Michaels of the Department of Microbiology and Immunology, Thomas Jefferson University for obtaining the information on the incidence of MS and gout. This work was supported, in part, by funds from the Biotechnology Foundation, Inc., which is supported by an appropriation from the State of Pennsylvania.
ABBREVIATIONS
- EAE
experimental allergic encephalomyelitis
- IFN-γ
interferon-γ
- MS
multiple sclerosis
- CNS
central nervous system
- LPS
lipopolysaccharide
- l-NMMA
n-methyl-l-arginine
- DHR123
dihydrorhodamine 123
- MBP
myelin basic protein
- CFA
complete Freund’s adjuvant
References
- 1.Koprowski H, Zheng Y M, Heber-Katz E, Fraser N, Rorke L, Fu Z F, Hanlon C, Dietzschold B. Proc Natl Acad Sci USA. 1993;90:3024–3027. doi: 10.1073/pnas.90.7.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin R F, Lin T-S, Tilton R G, Cross A H. J Exp Med. 1993;178:643–648. doi: 10.1084/jem.178.2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cross A H, Misko T P, Lin R F, Hickey W F, Trotter J L, Tilton R G. J Clin Invest. 1994;93:2684–2690. doi: 10.1172/JCI117282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang J, Dawson V L, Dawson T M, Snyder S H. Science. 1994;263:687–688. doi: 10.1126/science.8080500. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt H H H W, Walter U. Cell. 1994;78:919–925. doi: 10.1016/0092-8674(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 6.Akaike T, Weihe E, Schaefer MK-H, Fu Z F, Zheng Y M, Vogel W H, Schmidt H H H W, Koprowski H, Dietzschold B. J Neurovirol. 1995;1:118–125. doi: 10.3109/13550289509111016. [DOI] [PubMed] [Google Scholar]
- 7.Hooper D C, Ohnishi T S, Kean R, Numagami Y, Dietzschold B, Koprowski H. Proc Natl Acad Sci USA. 1995;92:5312–5316. doi: 10.1073/pnas.92.12.5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hooper D C, Bagasra O, Marini J C, Zborek A, Ohnishi S T, Kean R, Champion J M, Sarker A B, Bobroski L, Farber J L, Akaike T, Maeda H, Koprowski H. Proc Natl Acad Sci USA. 1997;94:2528–2533. doi: 10.1073/pnas.94.6.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akaike T, Noguchi Y, Suga M, Zheng Y M, Dietzschold B, Maeda H. Proc Natl Acad Sci USA. 1996;93:2448–2453. doi: 10.1073/pnas.93.6.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Radi R, Beckman J S, Bush K M, Freeman B A. Arch Biochem Biophys. 1991;288:481–487. doi: 10.1016/0003-9861(91)90224-7. [DOI] [PubMed] [Google Scholar]
- 11.Beckman J S, Ye Y Z, Anderson P G, Chen J, Accavitti M A, Tarpey M M, White C R. Biol Chem Hoppe-Seyler. 1994;375:81–88. doi: 10.1515/bchm3.1994.375.2.81. [DOI] [PubMed] [Google Scholar]
- 12.Ischiropoulos H, Zhu L, Chen J, Tsai M, Martin J, Smith C, Beckman J. Arch Biochem Biophys. 1992;298:431–437. doi: 10.1016/0003-9861(92)90431-u. [DOI] [PubMed] [Google Scholar]
- 13.Whiteman M, Halliwell B. Free Radical Res. 1996;25:275–283. doi: 10.3109/10715769609149052. [DOI] [PubMed] [Google Scholar]
- 14.Lorsbach R B, Murphy W J, Lowenstein C J, Snyder S H, Russell S W. J Biol Chem. 1993;268:1908–1913. [PubMed] [Google Scholar]
- 15.Duong T T, Finkelman F D, Singh B, Strejan G H. J Neuroimmunol. 1994;53:101–107. doi: 10.1016/0165-5728(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 16.Krakowski M, Owens T. Eur J Immunol. 1996;26:1641–1646. doi: 10.1002/eji.1830260735. [DOI] [PubMed] [Google Scholar]
- 17.Green L C, Wagner D A, Glogowski J, Skipper P L, Wishnok J S, Tannenbaum S R. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 18.Trost L C, Lemasters J J. Anal Biochem. 1994;220:149–153. doi: 10.1006/abio.1994.1311. [DOI] [PubMed] [Google Scholar]
- 19.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel R M, Aguet M. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 20.Deibler G E, Martenson R E, Kies M W. Prep Biochem. 1972;2:139–165. doi: 10.1080/00327487208061467. [DOI] [PubMed] [Google Scholar]
- 21.Jelic-Ivanovic Z, Spasic S, Majkic-Singh N, Todorovic P. Clin Chemistry. 1985;31:1141–1143. [PubMed] [Google Scholar]
- 22.Langford H G, Blaufox M D, Borhani N O, Curb J D, Molteni A, Schneider K A, Pressel S. Arch Intern Med. 1987;147:645–649. doi: 10.1001/archinte.147.4.645. [DOI] [PubMed] [Google Scholar]
- 23.Mikkelsen W M, Dodge H J, Valkenburg H. Am J Med. 1965;39:242–251. doi: 10.1016/0002-9343(65)90048-3. [DOI] [PubMed] [Google Scholar]
- 24.Zhao W, Tilton R G, Corbett J A, McDaniel M L, Misko T P, Williamson J R, Cross A H, Hickey W F. J Neuroimmunol. 1996;64:123–133. doi: 10.1016/0165-5728(95)00158-1. [DOI] [PubMed] [Google Scholar]
- 25.Brenner T, Brocke S, Szafer F, Sobel R A, Parkinson J F, Perez D H, Steinman L. J Immunol. 1997;158:2940–2946. [PubMed] [Google Scholar]
- 26.Beckman J S. J Dev Physiol. 1991;15:53–59. [PubMed] [Google Scholar]
- 27.Boullerne A I, Petry K G, Meynard M, Geffard M. J Neuroimmunol. 1995;60:117–124. doi: 10.1016/0165-5728(95)00061-6. [DOI] [PubMed] [Google Scholar]
- 28.Bolanos J P, Heales S J, Land J M, Clark J B. J Neurochem. 1995;64:1965–1972. doi: 10.1046/j.1471-4159.1995.64051965.x. [DOI] [PubMed] [Google Scholar]
- 29.Chou, S. M. Wang, H. S. & Tanaguchi, A. (1996) J. Neurol. Sci. 139, Suppl., 16–26. [DOI] [PubMed]
- 30.Newcombe J, Li H, Cuzner M L. Neuropathol Appl Neurobiol. 1994;20:152–162. doi: 10.1111/j.1365-2990.1994.tb01174.x. [DOI] [PubMed] [Google Scholar]
- 31.Shibutani S, Takeshita M, Grollman A P. Nature (London) 1991;349:431–434. doi: 10.1038/349431a0. [DOI] [PubMed] [Google Scholar]
- 32.Inoue S, Kawanishi S. FEBS Lett. 1995;371:86–88. doi: 10.1016/0014-5793(95)00873-8. [DOI] [PubMed] [Google Scholar]
- 33.Douki T, Cadet J. Free Radical Res. 1996;24:369–380. doi: 10.3109/10715769609088035. [DOI] [PubMed] [Google Scholar]
- 34.Kennedy L J, Moore K, Jr, Caulfield J L, Tannenbaum S R, Dedon P C. Chem Res Toxicol. 1997;10:386–392. doi: 10.1021/tx960102w. [DOI] [PubMed] [Google Scholar]
- 35.Dowling P, Husar W, Mennona J, Donnenfeld H, Cook S, Sidhu M. J Neurol Sci. 1997;149:1–11. doi: 10.1016/s0022-510x(97)05213-1. [DOI] [PubMed] [Google Scholar]
- 36.Sabelko K A, Kelly K A, Nahm M H, Cross A H, Russell J H. J Immunol. 1997;159:3096–3099. [PubMed] [Google Scholar]
- 37.Waldner H, Sobel R A, Howard E, Kuchroo V K. J Immunol. 1997;159:3100–3103. [PubMed] [Google Scholar]
- 38.Good P F, Werner P, Hsu A, Olanow C W, Perl D P. Am J Pathol. 1996;149:21–28. [PMC free article] [PubMed] [Google Scholar]
- 39.Smith M A, Richey-Harris P L, Sayre L M, Beckman J S, Perry G. J Neurosci. 1997;17:2653–2657. doi: 10.1523/JNEUROSCI.17-08-02653.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kamijo R, Shapiro D, Le J, Huang S, Aguet M, Vilcek J. Proc Natl Acad Sci USA. 1993;90:6626–6630. doi: 10.1073/pnas.90.14.6626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hellsten Y, Tullson P C, Richter E A, Bangsbo J. Free Radical Biol Med. 1997;22:169–174. doi: 10.1016/s0891-5849(96)00286-9. [DOI] [PubMed] [Google Scholar]