Abstract
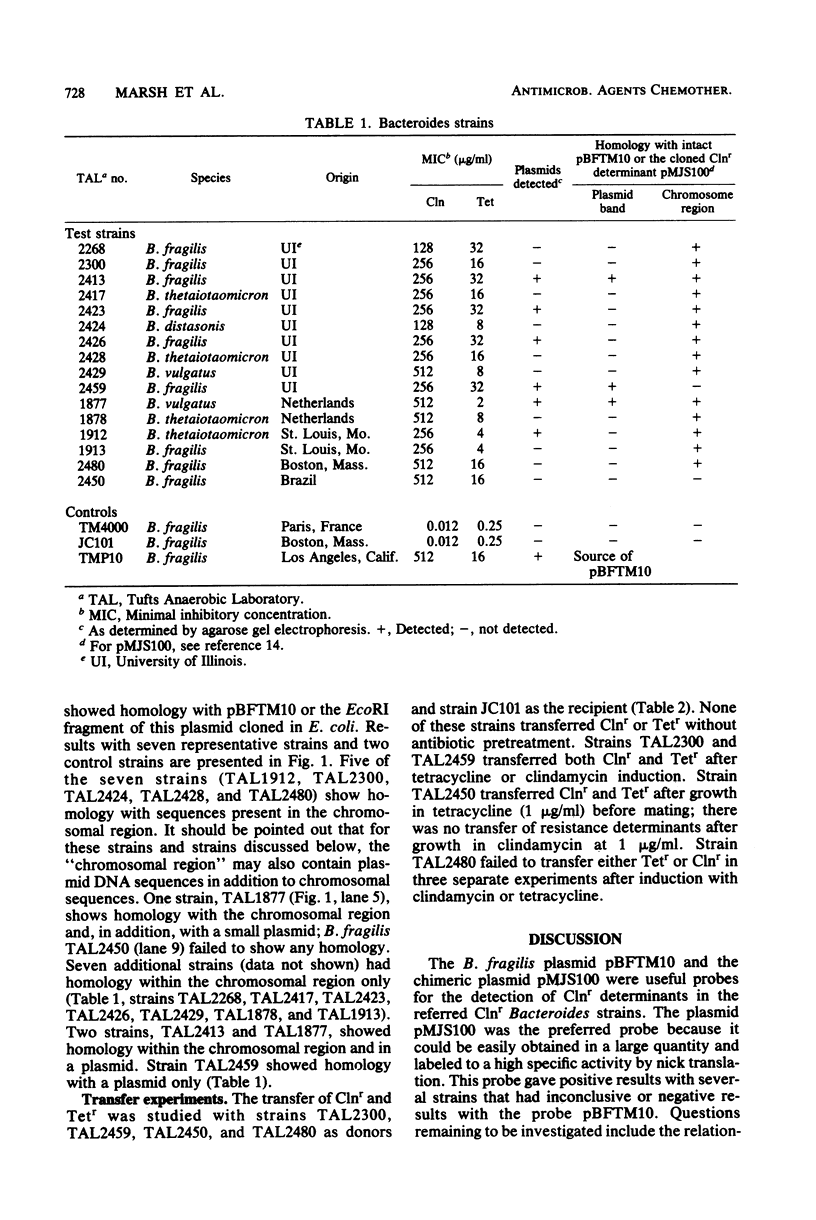
The sequence homology of clindamycin resistance (Clnr) determinants was studied in 16 Clnr Bacteroides strains. The isolates were surveyed for plasmid content, homology with the Clnr determinant of pBFTM10, and ability to transfer Clnr. The Clnr DNA probes used in the Southern hybridizations were pBFTM10 and a plasmid derivative containing an EcoRI fragment of pBFTM10 cloned into Escherichia coli. A total of 13 of 16 Clnr strains also carried tetracycline resistance, and 15 of 16 Clnr Bacteroides isolates showed homology with the Clnr determinant of pBFTM10. These data suggest that the previously characterized Clnr determinant of pBFTM10 is widely distributed in nature and may be found on either a plasmid or the chromosome. The Clnr Bacteroides fragilis strain which lacked homology with pBFTM10 also had different transfer properties; thus, more than one type of Clnr determinant may exist in Bacteroides spp.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bawdon R. E., Rozmiej E., Palchaudhuri S., Krakowiak J. Variability in the susceptibility pattern of Bacteroides fragilis in four Detroit area hospitals. Antimicrob Agents Chemother. 1979 Nov;16(5):664–666. doi: 10.1128/aac.16.5.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Chou J., Cohen S. N. In vitro gene fusions that join an enzymatically active beta-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J Bacteriol. 1980 Aug;143(2):971–980. doi: 10.1128/jb.143.2.971-980.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbach S. L., Bartlett J. G. Anaerobic infections. 1. N Engl J Med. 1974 May 23;290(21):1177–1184. doi: 10.1056/NEJM197405232902106. [DOI] [PubMed] [Google Scholar]
- Magot M., Fayolle F., Privitera G., Sebald M. Transposon-like structures in the Bacteroides fragilis MLS plasmid plP 410. Mol Gen Genet. 1981;181(4):559–561. doi: 10.1007/BF00428754. [DOI] [PubMed] [Google Scholar]
- Malamy M. H., Tally F. P. Mechanisms of drug-resistance transfer in Bacteroides fragilis. J Antimicrob Chemother. 1981 Dec;8 (Suppl 500):59–75. doi: 10.1093/jac/8.suppl_d.59. [DOI] [PubMed] [Google Scholar]
- Marrie T. J., Haldane E. V., Swantee C. A., Kerr E. A. Susceptibility of anaerobic bacteria to nine antimicrobial agents and demonstration of decreased susceptibility of Clostridium perfringens to penicillin. Antimicrob Agents Chemother. 1981 Jan;19(1):51–55. doi: 10.1128/aac.19.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privitera G., Dublanchet A., Sebald M. Transfer of multiple antibiotic resistance between subspecies of Bacteroides fragilis. J Infect Dis. 1979 Jan;139(1):97–101. doi: 10.1093/infdis/139.1.97. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Shimell M. J., Smith C. J., Tally F. P., Macrina F. L., Malamy M. H. Hybridization studies reveal homologies between pBF4 and pBFTM10, Two clindamycin-erythromycin resistance transfer plasmids of Bacteroides fragilis. J Bacteriol. 1982 Nov;152(2):950–953. doi: 10.1128/jb.152.2.950-953.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tally F. P., Snydman D. R., Shimell M. J., Malamy M. H. Characterization of pBFTM10, a clindamycin-erythromycin resistance transfer factor from Bacteroides fragilis. J Bacteriol. 1982 Aug;151(2):686–691. doi: 10.1128/jb.151.2.686-691.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tally F. P., Sosa A., Jacobus N. V., Malamy M. H. Clindamycin resistance in Bacteroides fragilis. J Antimicrob Chemother. 1981 Dec;8 (Suppl 500):43–48. doi: 10.1093/jac/8.suppl_d.43. [DOI] [PubMed] [Google Scholar]
- Thadepalli H., Gorbach S. L., Broido P. W., Norsen J., Nyhus L. Abdominal trauma, anaerobes, and antibiotics. Surg Gynecol Obstet. 1973 Aug;137(2):270–276. [PubMed] [Google Scholar]
- Tomich P. K., An F. Y., Clewell D. B. Properties of erythromycin-inducible transposon Tn917 in Streptococcus faecalis. J Bacteriol. 1980 Mar;141(3):1366–1374. doi: 10.1128/jb.141.3.1366-1374.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisblum B., Holder S. B., Halling S. M. Deoxyribonucleic acid sequence common to staphylococcal and streptococcal plasmids which specify erythromycin resistance. J Bacteriol. 1979 Jun;138(3):990–998. doi: 10.1128/jb.138.3.990-998.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch R. A., Jones K. R., Macrina F. L. Transferable lincosamide-macrolide resistance in Bacteroides. Plasmid. 1979 Apr;2(2):261–268. doi: 10.1016/0147-619x(79)90044-1. [DOI] [PubMed] [Google Scholar]
- Welch R. A., Macrina F. L. Physical characterization of Bacteroides fragilis R plasmid pBF4. J Bacteriol. 1981 Feb;145(2):867–872. doi: 10.1128/jb.145.2.867-872.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]



