Abstract
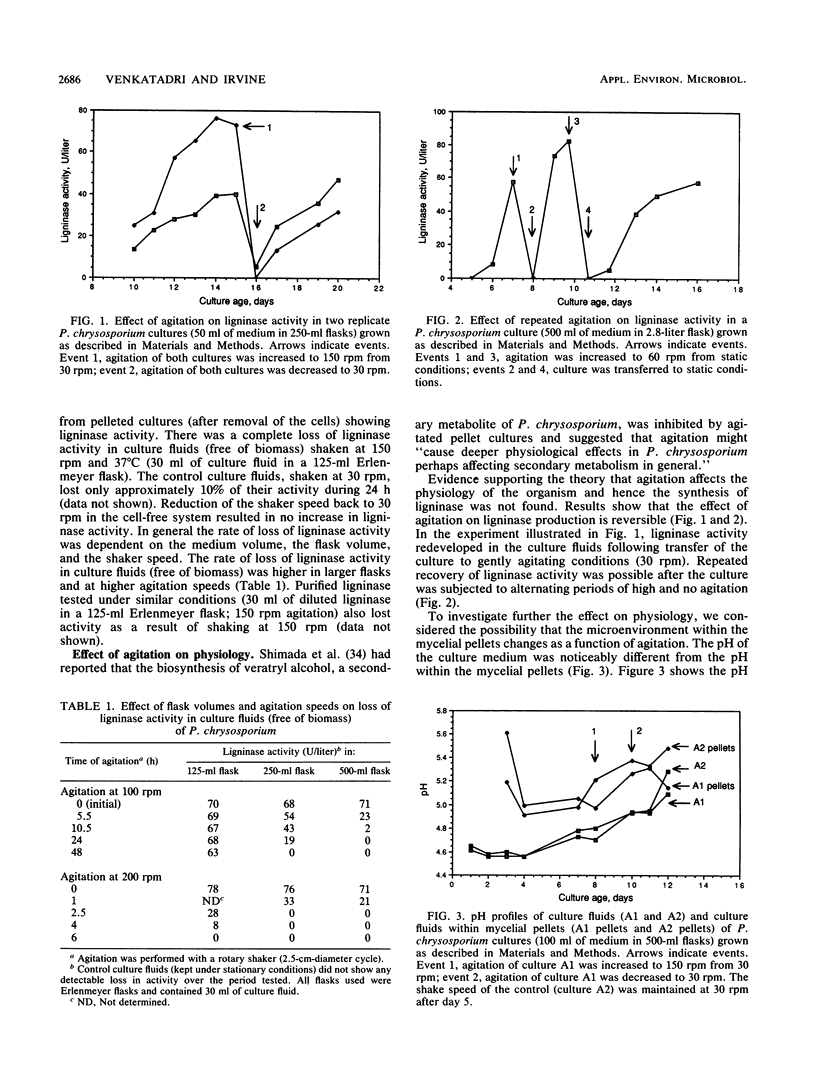
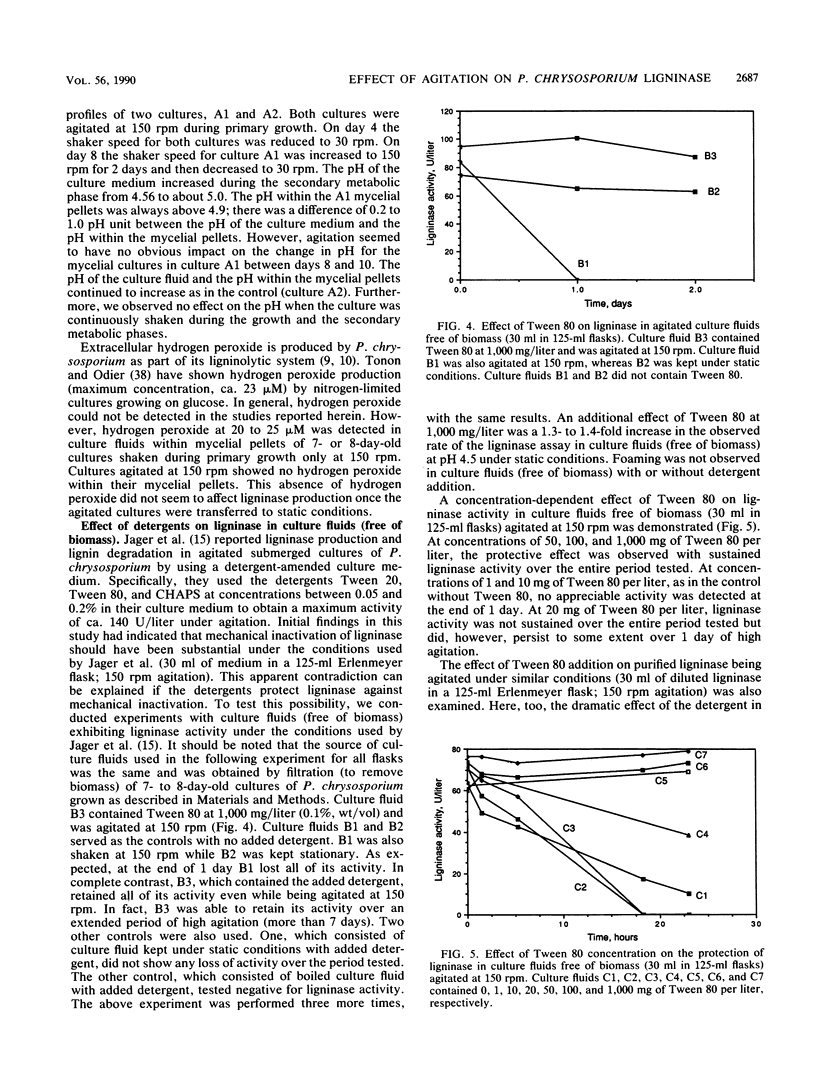
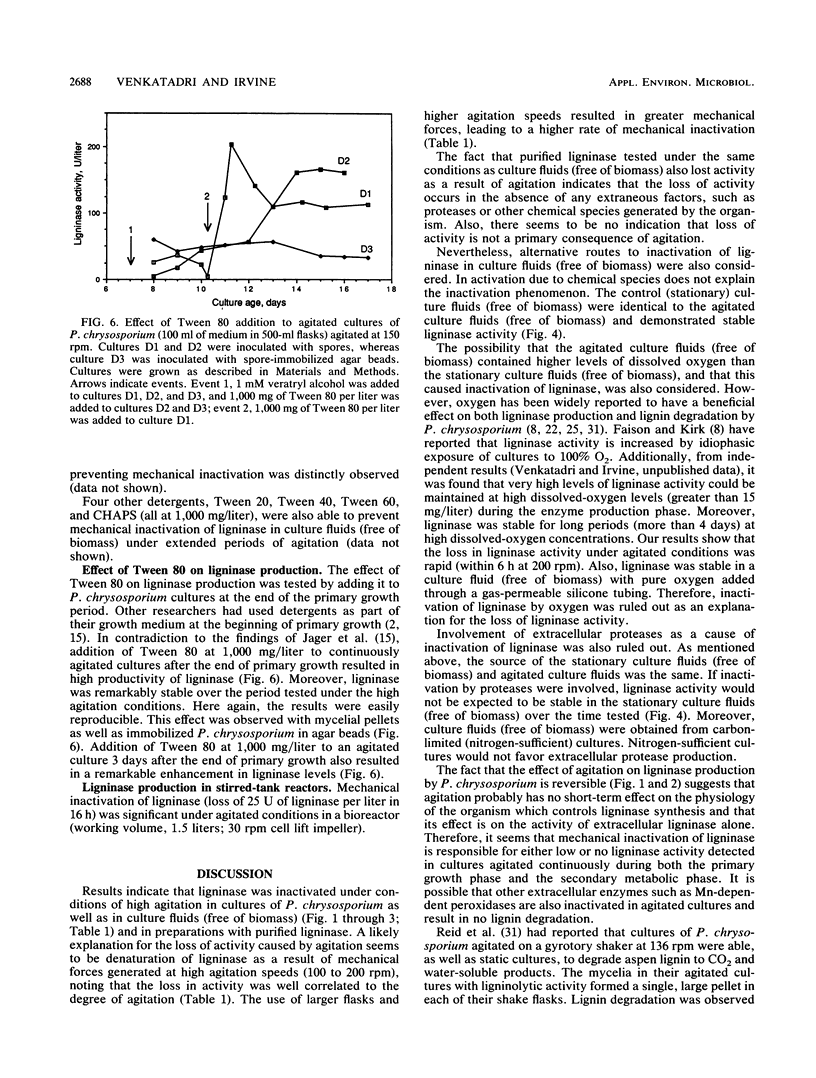
The white rot fungus Phanerochaete chrysosporium produces extracellular ligninases as part of its idiophasic ligninolytic system. Agitation has been widely reported to suppress both ligninase production and lignin degradation. Results show that mechanical inactivation of ligninase is possibly the reason why ligninase accumulation is low or absent in agitated shake-flask cultures. Agitation seems to affect the catalytic activity of ligninase and has no apparent effect on either the rate of ligninase production or the physiology of P. chrysosporium. The detergents Tween 20, Tween 40, Tween 60, Tween 80, and 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS) are able to protect both purified ligninase and extant ligninase in culture fluids (free of biomass) against mechanical inactivation due to agitation. Addition of Tween 80 at the end of primary growth to agitated shake flasks containing either pelleted or immobilized mycelial cultures results in production and maintenance of high levels of ligninase activity over several days under conditions of high agitation. Possible mechanisms by which the detergents could protect ligninase are discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borgström B., Erlanson C. Pancreatic lipase and co-lipase. Interactions and effects of bile salts and other detergents. Eur J Biochem. 1973 Aug 1;37(1):60–68. doi: 10.1111/j.1432-1033.1973.tb02957.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Canioni P., Julien R., Rathelot J., Sarda L. Interaction of porcine pancreatic colipase with a nonionic detergent, Triton X-100: spectrophotometric studies. Lipids. 1980 Jan;15(1):6–9. doi: 10.1007/BF02534109. [DOI] [PubMed] [Google Scholar]
- Faison B. D., Kirk T. K. Factors Involved in the Regulation of a Ligninase Activity in Phanerochaete chrysosporium. Appl Environ Microbiol. 1985 Feb;49(2):299–304. doi: 10.1128/aem.49.2.299-304.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faison B. D., Kirk T. K. Relationship Between Lignin Degradation and Production of Reduced Oxygen Species by Phanerochaete chrysosporium. Appl Environ Microbiol. 1983 Nov;46(5):1140–1145. doi: 10.1128/aem.46.5.1140-1145.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forney L. J., Reddy C. A., Pankratz H. S. Ultrastructural Localization of Hydrogen Peroxide Production in Ligninolytic Phanerochaete chrysosporium Cells. Appl Environ Microbiol. 1982 Sep;44(3):732–736. doi: 10.1128/aem.44.3.732-736.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forney L. J., Reddy C. A., Tien M., Aust S. D. The involvement of hydroxyl radical derived from hydrogen peroxide in lignin degradation by the white rot fungus Phanerochaete chrysosporium. J Biol Chem. 1982 Oct 10;257(19):11455–11462. [PubMed] [Google Scholar]
- Glenn J. K., Morgan M. A., Mayfield M. B., Kuwahara M., Gold M. H. An extracellular H2O2-requiring enzyme preparation involved in lignin biodegradation by the white rot basidiomycete Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1983 Aug 12;114(3):1077–1083. doi: 10.1016/0006-291x(83)90672-1. [DOI] [PubMed] [Google Scholar]
- Haemmerli S. D., Leisola M. S., Sanglard D., Fiechter A. Oxidation of benzo(a)pyrene by extracellular ligninases of Phanerochaete chrysosporium. Veratryl alcohol and stability of ligninase. J Biol Chem. 1986 May 25;261(15):6900–6903. [PubMed] [Google Scholar]
- Hammel K. E., Kalyanaraman B., Kirk T. K. Oxidation of polycyclic aromatic hydrocarbons and dibenzo[p]-dioxins by Phanerochaete chrysosporium ligninase. J Biol Chem. 1986 Dec 25;261(36):16948–16952. [PubMed] [Google Scholar]
- Jeffries T. W., Choi S., Kirk T. K. Nutritional Regulation of Lignin Degradation by Phanerochaete chrysosporium. Appl Environ Microbiol. 1981 Aug;42(2):290–296. doi: 10.1128/aem.42.2.290-296.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäger A., Croan S., Kirk T. K. Production of Ligninases and Degradation of Lignin in Agitated Submerged Cultures of Phanerochaete chrysosporium. Appl Environ Microbiol. 1985 Nov;50(5):1274–1278. doi: 10.1128/aem.50.5.1274-1278.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempner D. H., Johnson B. J. Lipid-protein interactions: enhancement of enzyme activity of L-glutamic acid dehydrogenase by nonionic detergents. J Pharm Sci. 1976 Dec;65(12):1799–1801. doi: 10.1002/jps.2600651226. [DOI] [PubMed] [Google Scholar]
- Keyser P., Kirk T. K., Zeikus J. G. Ligninolytic enzyme system of Phanaerochaete chrysosporium: synthesized in the absence of lignin in response to nitrogen starvation. J Bacteriol. 1978 Sep;135(3):790–797. doi: 10.1128/jb.135.3.790-797.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leatham G. F., Crawford R. L., Kirk T. K. Degradation of Phenolic Compounds and Ring Cleavage of Catechol by Phanerochaete chrysosporium. Appl Environ Microbiol. 1983 Jul;46(1):191–197. doi: 10.1128/aem.46.1.191-197.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese E. T., Maguire A. Surfactants as stimulants of enzyme production by microorganisms. Appl Microbiol. 1969 Feb;17(2):242–245. doi: 10.1128/am.17.2.242-245.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renganathan V., Miki K., Gold M. H. Multiple molecular forms of diarylpropane oxygenase, an H2O2-requiring, lignin-degrading enzyme from Phanerochaete chrysosporium. Arch Biochem Biophys. 1985 Aug 15;241(1):304–314. doi: 10.1016/0003-9861(85)90387-x. [DOI] [PubMed] [Google Scholar]
- Seibles T. S. Interaction of dodecyl sulfate with native and modified beta-lactoglobulin. Biochemistry. 1969 Jul;8(7):2949–2954. doi: 10.1021/bi00835a039. [DOI] [PubMed] [Google Scholar]
- Tien M., Kirk T. K. Lignin-Degrading Enzyme from the Hymenomycete Phanerochaete chrysosporium Burds. Science. 1983 Aug 12;221(4611):661–663. doi: 10.1126/science.221.4611.661. [DOI] [PubMed] [Google Scholar]
- Tien M., Kirk T. K. Lignin-degrading enzyme from Phanerochaete chrysosporium: Purification, characterization, and catalytic properties of a unique H(2)O(2)-requiring oxygenase. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2280–2284. doi: 10.1073/pnas.81.8.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonon F., Odier E. Influence of Veratryl Alcohol and Hydrogen Peroxide on Ligninase Activity and Ligninase Production by Phanerochaete chrysosporium. Appl Environ Microbiol. 1988 Feb;54(2):466–472. doi: 10.1128/aem.54.2.466-472.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


