Abstract
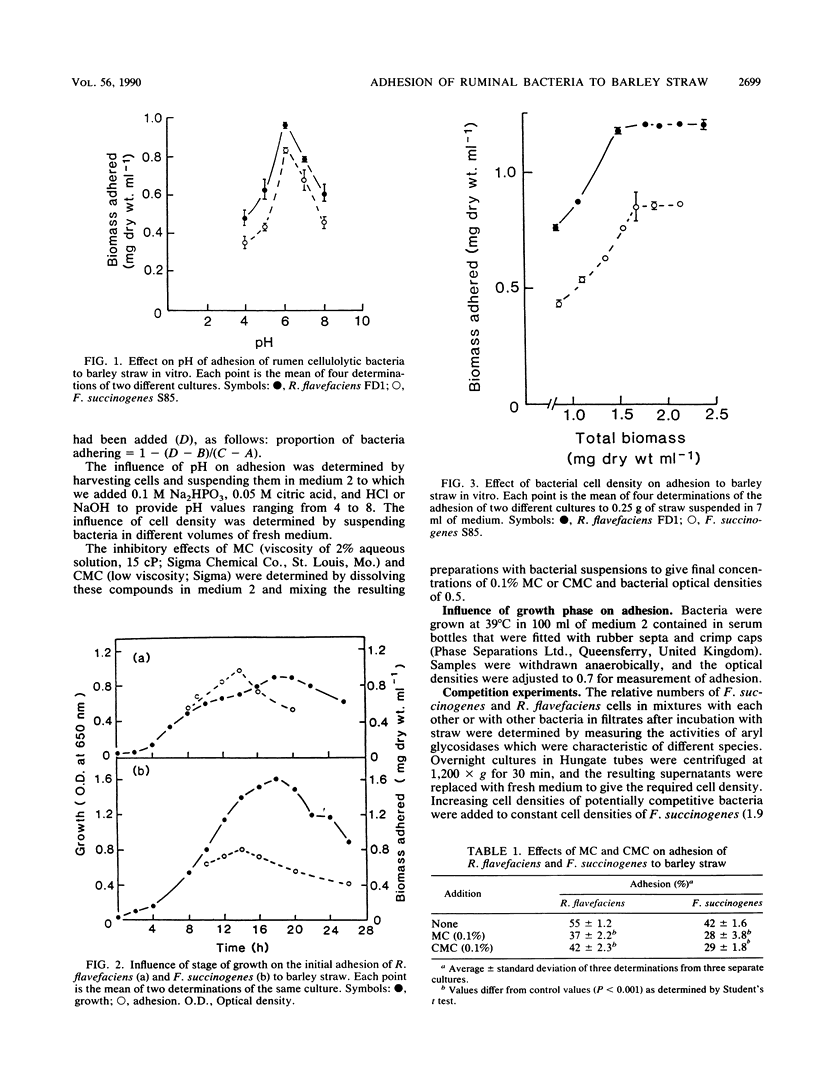
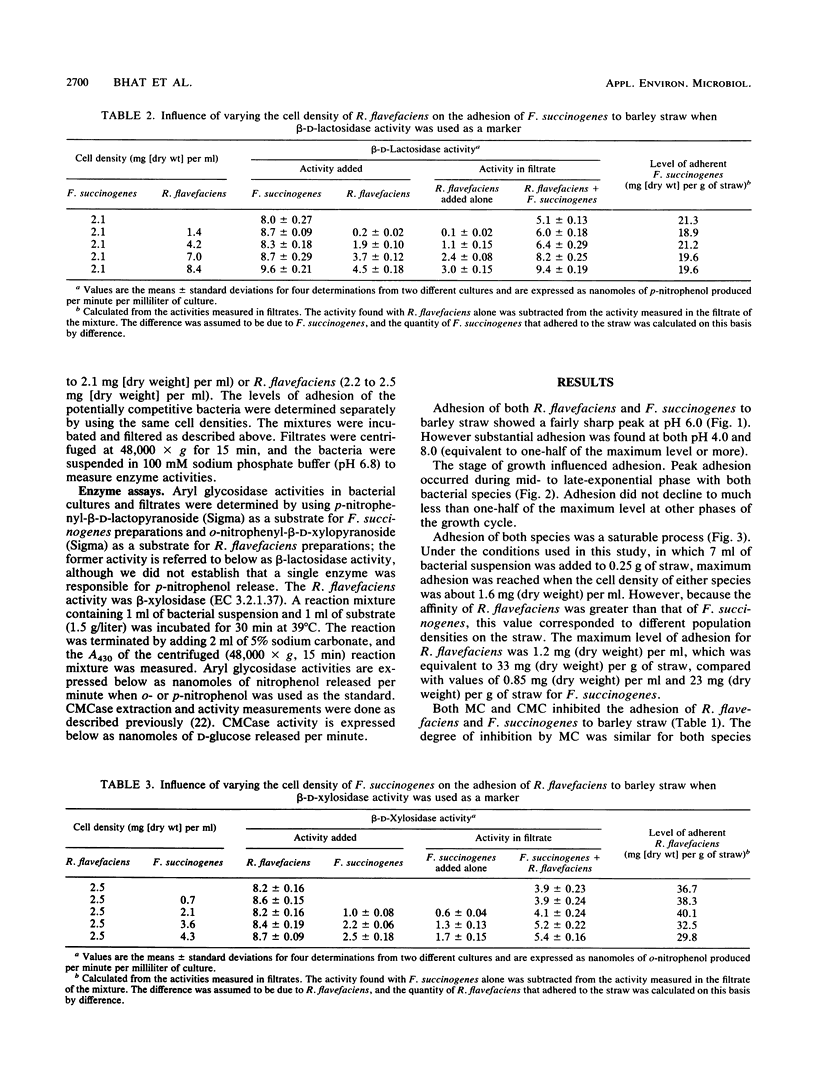
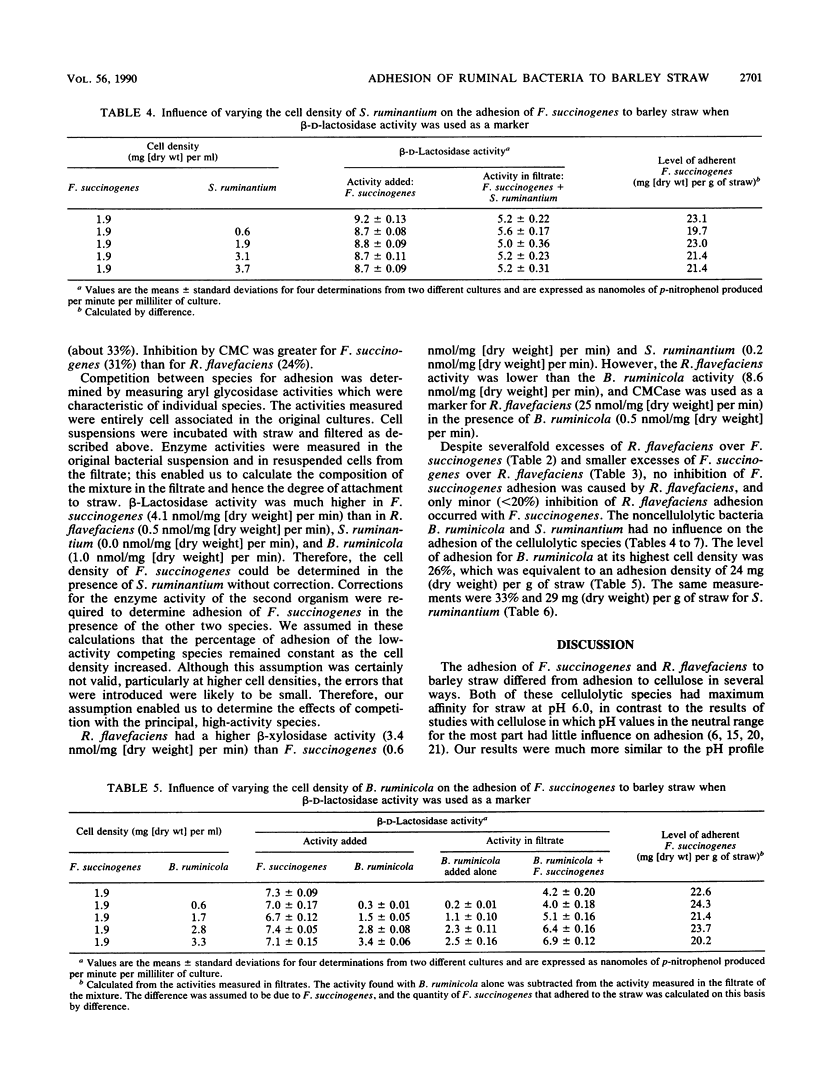
Adhesion of the cellulolytic ruminal bacteria Ruminococcus flavefaciens and Fibrobacter succinogenes to barley straw was measured by incubating bacterial suspensions with hammer-milled straw for 30 min, filtering the mixtures through sintered glass filters, and measuring the optical densities of the filtrates. Maximum adhesion of both species occurred at pH 6.0 and during mid- to late-exponential phase. Adhesion was saturable at 33 and 23 mg (dry weight) g of straw−1 for R. flavefaciens and F. succinogenes, respectively. Methyl cellulose and carboxymethyl cellulose inhibited adhesion by 24 to 33%. Competition between species was determined by measuring characteristic cell-associated enzyme activities in filtrates of mixtures incubated with straw; p-nitrophenyl-β-d-lactopyranoside hydrolysis was used as a marker for F. succinogenes, while either β-xylosidase or carboxymethyl cellulase was used for R. flavefaciens, depending on the other species present. R. flavefaciens had no influence on F. succinogenes adhesion, and F. succinogenes had only a minor (<20%) effect on R. flavefaciens adhesion. The noncellulolytic ruminal bacteria Bacteroides ruminicola and Selenomonas ruminantium had no influence on adhesion of either cellulolytic species, although these organisms also adhered to the straw. We concluded that R. flavefaciens and F. succinogenes have separate, specific adhesion sites on barley straw that are not obscured by competition with non-cellulolytic species.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amos H. E., Akin D. E. Rumen protozoal degradation of structurally intact forage tissues. Appl Environ Microbiol. 1978 Sep;36(3):513–522. doi: 10.1128/aem.36.3.513-522.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Moreno E. C., Spinell D. M. Model delineating the effects of a salivary pellicle on the adsorption of Streptococcus miteor onto hydroxyapatite. Infect Immun. 1976 Oct;14(4):1109–1112. doi: 10.1128/iai.14.4.1109-1112.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J., Forsberg C. W. Factors affecting adhesion of Fibrobacter succinogenes subsp. succinogenes S85 and adherence-defective mutants to cellulose. Appl Environ Microbiol. 1989 Dec;55(12):3039–3044. doi: 10.1128/aem.55.12.3039-3044.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groleau D., Forsberg C. W. Partial characterization of the extracellular carboxymethylcellulase activity produced by the rumen bacterium Bacteroides succinogenes. Can J Microbiol. 1983 May;29(5):504–517. doi: 10.1139/m83-080. [DOI] [PubMed] [Google Scholar]
- Kopecný J., Jurcuk J. F., Bartos S. The effect of pH and 1,4-dithiothreitol on the adhesion of rumen bacteria. Folia Microbiol (Praha) 1983;28(2):130–133. doi: 10.1007/BF02877369. [DOI] [PubMed] [Google Scholar]
- Kudo H., Cheng K. J., Costerton J. W. Electron microscopic study of the methylcellulose-mediated detachment of cellulolytic rumen bacteria from cellulose fibers. Can J Microbiol. 1987 Mar;33(3):267–272. doi: 10.1139/m87-045. [DOI] [PubMed] [Google Scholar]
- Latham M. J., Brooker B. E., Pettipher G. L., Harris P. J. Adhesion of Bacteroides succinogenes in pure culture and in the presence of Ruminococcus flavefaciens to cell walls in leaves of perennial ryegrass (Lolium perenne). Appl Environ Microbiol. 1978 Jun;35(6):1166–1173. doi: 10.1128/aem.35.6.1166-1173.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latham M. J., Brooker B. E., Pettipher G. L., Harris P. J. Ruminococcus flavefaciens Cell Coat and Adhesion to Cotton Cellulose and to Cell Walls in Leaves of Perennial Ryegrass (Lolium perenne). Appl Environ Microbiol. 1978 Jan;35(1):156–165. doi: 10.1128/aem.35.1.156-165.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson H., Irvin R., Costerton J. W., Cheng K. J. Ultrastructure and adhesion properties of Ruminococcus albus. J Bacteriol. 1975 Apr;122(1):278–287. doi: 10.1128/jb.122.1.278-287.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen M. A., Hespell R. B., White B. A., Bothast R. J. Inhibitory Effects of Methylcellulose on Cellulose Degradation by Ruminococcus flavefaciens. Appl Environ Microbiol. 1988 Apr;54(4):890–897. doi: 10.1128/aem.54.4.890-897.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen M. A., White B. A., Hespell R. B. Improved assay for quantitating adherence of ruminal bacteria to cellulose. Appl Environ Microbiol. 1989 Aug;55(8):2089–2091. doi: 10.1128/aem.55.8.2089-2091.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva A. T., Wallace R. J., Orskov E. R. Use of particle-bound microbial enzyme activity to predict the rate and extent of fibre degradation in the rumen. Br J Nutr. 1987 May;57(3):407–415. doi: 10.1079/bjn19870048. [DOI] [PubMed] [Google Scholar]
- Stewart C. S. Factors affecting the cellulolytic activity of rumen contents. Appl Environ Microbiol. 1977 Mar;33(3):497–502. doi: 10.1128/aem.33.3.497-502.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White B. A., Rasmussen M. A., Gardner R. M. Methylcellulose inhibition of exo-beta-1,4-glucanase A from Ruminococcus flavefaciens FD-1. Appl Environ Microbiol. 1988 Jun;54(6):1634–1636. doi: 10.1128/aem.54.6.1634-1636.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windham W. R., Akin D. E. Rumen fungi and forage fiber degradation. Appl Environ Microbiol. 1984 Sep;48(3):473–476. doi: 10.1128/aem.48.3.473-476.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


