Abstract
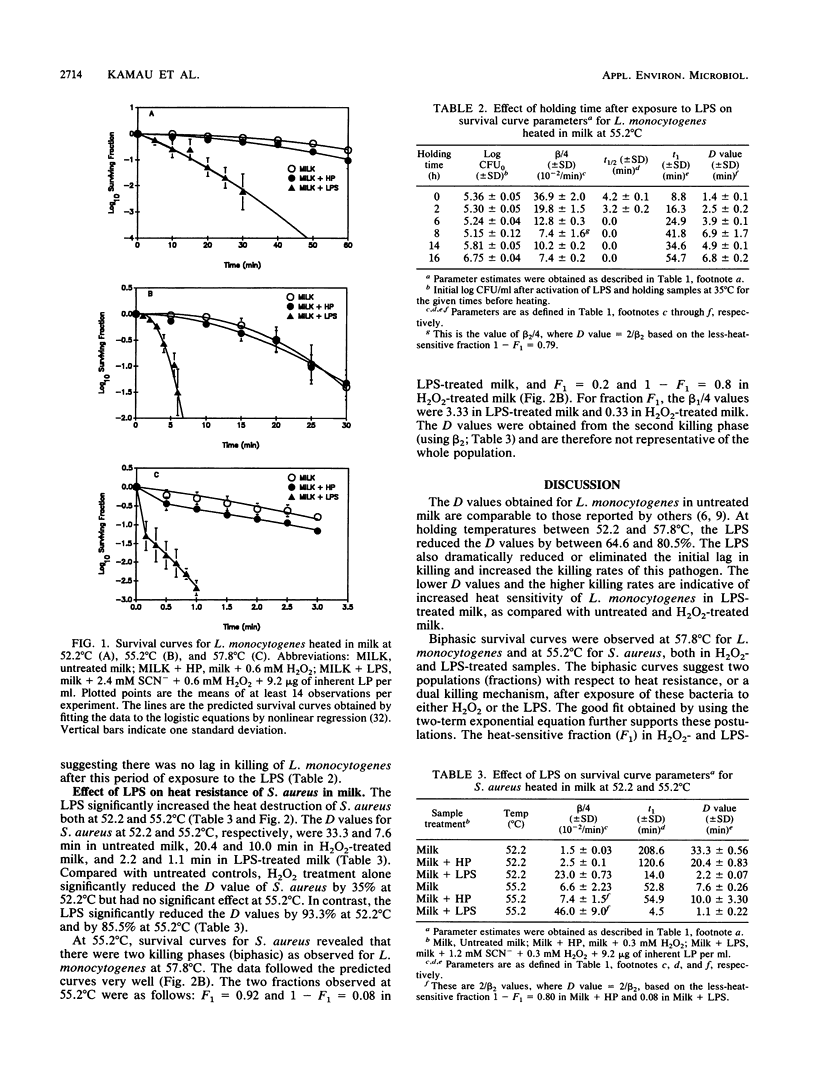
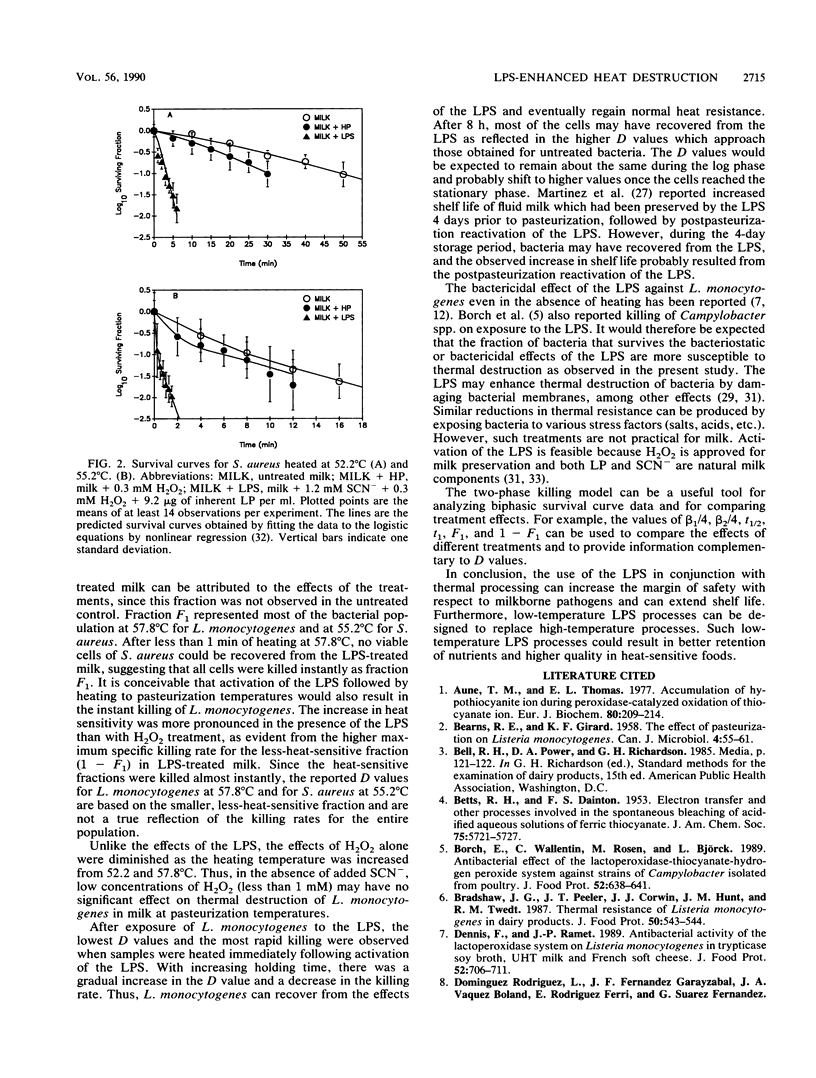
The lactoperoxidase system (LPS) enhanced thermal destruction of Listeria monocytogenes and Staphylococcus aureus. After LPS activation, biphasic survival curves were observed for L. monocytogenes at 57.8 degrees C and for S. aureus at 55.2 degrees C. The data were consistent with a model that assumed two bacterial populations differing in heat sensitivity. The more heat-sensitive fractions (93% of the L. monocytogenes, 92% of the S. aureus) were killed almost instantly. For these biphasic survival curves, D values were based on the much smaller, less-heat-sensitive fractions. For L. monocytogenes, the D52.2 degrees C values were 30.2 min (untreated milk) and 10.7 min (LPS activated); corresponding D55.2 degrees C values were 8.2 and 1.6 min; corresponding D57.8 degrees C values were 2.3 and 0.5 min. For S. aureus, the D52.2 degrees C values were 33.3 min (untreated milk) and 2.2 min (LPS activated), and the corresponding D55.2 degrees C values were 7.6 and 1.1 min, respectively. The most rapid killing of L. monocytogenes occurred when samples were heated soon after activation of the LPS. Activation of the LPS followed by heating can increase the margin of safety with respect to milkborne pathogens.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aune T. M., Thomas E. L. Accumulation of hypothiocyanite ion during peroxidase-catalyzed oxidation of thiocyanate ion. Eur J Biochem. 1977 Oct 17;80(1):209–214. doi: 10.1111/j.1432-1033.1977.tb11873.x. [DOI] [PubMed] [Google Scholar]
- BEARNS R. E., GIRARD K. F. The effect of pasteurization on Listeria monocytogenes. Can J Microbiol. 1958 Feb;4(1):55–61. doi: 10.1139/m58-007. [DOI] [PubMed] [Google Scholar]
- Doyle M. P., Glass K. A., Beery J. T., Garcia G. A., Pollard D. J., Schultz R. D. Survival of Listeria monocytogenes in milk during high-temperature, short-time pasteurization. Appl Environ Microbiol. 1987 Jul;53(7):1433–1438. doi: 10.1128/aem.53.7.1433-1438.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez Garayzabal J. F., Dominguez Rodriguez L., Vazquez Boland J. A., Rodriguez Ferri E. F., Briones Dieste V., Blanco Cancelo J. L., Suarez Fernandez G. Survival of Listeria monocytogenes in raw milk treated in a pilot plant size pasteurizer. J Appl Bacteriol. 1987 Dec;63(6):533–537. doi: 10.1111/j.1365-2672.1987.tb02723.x. [DOI] [PubMed] [Google Scholar]
- Fleming D. W., Cochi S. L., MacDonald K. L., Brondum J., Hayes P. S., Plikaytis B. D., Holmes M. B., Audurier A., Broome C. V., Reingold A. L. Pasteurized milk as a vehicle of infection in an outbreak of listeriosis. N Engl J Med. 1985 Feb 14;312(7):404–407. doi: 10.1056/NEJM198502143120704. [DOI] [PubMed] [Google Scholar]
- Gray M. L., Killinger A. H. Listeria monocytogenes and listeric infections. Bacteriol Rev. 1966 Jun;30(2):309–382. doi: 10.1128/br.30.2.309-382.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnan M. J., Mascola L., Lou X. D., Goulet V., May S., Salminen C., Hird D. W., Yonekura M. L., Hayes P., Weaver R. Epidemic listeriosis associated with Mexican-style cheese. N Engl J Med. 1988 Sep 29;319(13):823–828. doi: 10.1056/NEJM198809293191303. [DOI] [PubMed] [Google Scholar]
- Mansson-Rahemtulla B., Baldone D. C., Pruitt K. M., Rahemtulla F. Specific assays for peroxidases in human saliva. Arch Oral Biol. 1986;31(10):661–668. doi: 10.1016/0003-9969(86)90095-6. [DOI] [PubMed] [Google Scholar]
- Mickelson M. N. Glucose transport in Streptococcus agalactiae and its inhibition by lactoperoxidase-thiocyanate-hydrogen peroxide. J Bacteriol. 1977 Nov;132(2):541–548. doi: 10.1128/jb.132.2.541-548.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlech W. F., 3rd, Lavigne P. M., Bortolussi R. A., Allen A. C., Haldane E. V., Wort A. J., Hightower A. W., Johnson S. E., King S. H., Nicholls E. S. Epidemic listeriosis--evidence for transmission by food. N Engl J Med. 1983 Jan 27;308(4):203–206. doi: 10.1056/NEJM198301273080407. [DOI] [PubMed] [Google Scholar]
- Shindler J. S., Childs R. E., Bardsley W. G. Peroxidase from human cervical mucus. The isolation and characterisation. Eur J Biochem. 1976 Jun 1;65(2):325–331. doi: 10.1111/j.1432-1033.1976.tb10345.x. [DOI] [PubMed] [Google Scholar]
- Thomas E. L., Aune T. M. Lactoperoxidase, peroxide, thiocyanate antimicrobial system: correlation of sulfhydryl oxidation with antimicrobial action. Infect Immun. 1978 May;20(2):456–463. doi: 10.1128/iai.20.2.456-463.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins P. O., Bourgeois R., Murray R. G. Psychrotrophic properties of Listeria monocytogenes. Can J Microbiol. 1972 May;18(5):543–551. doi: 10.1139/m72-087. [DOI] [PubMed] [Google Scholar]


