Abstract
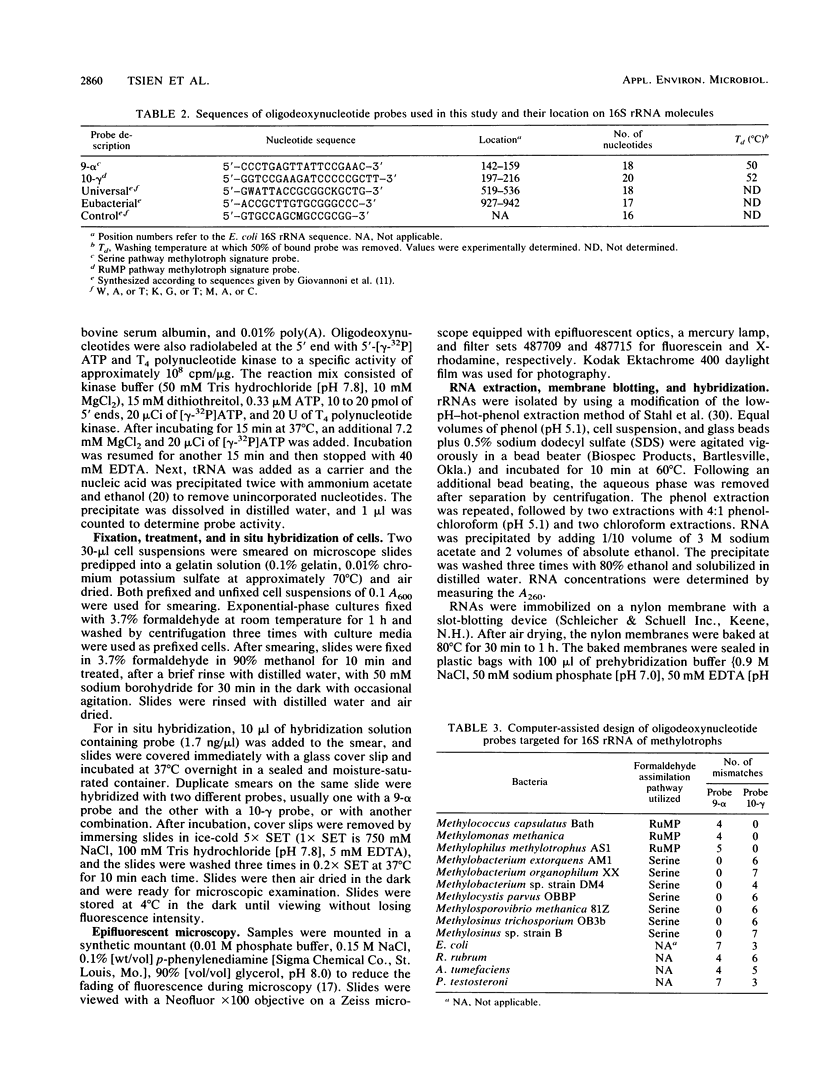
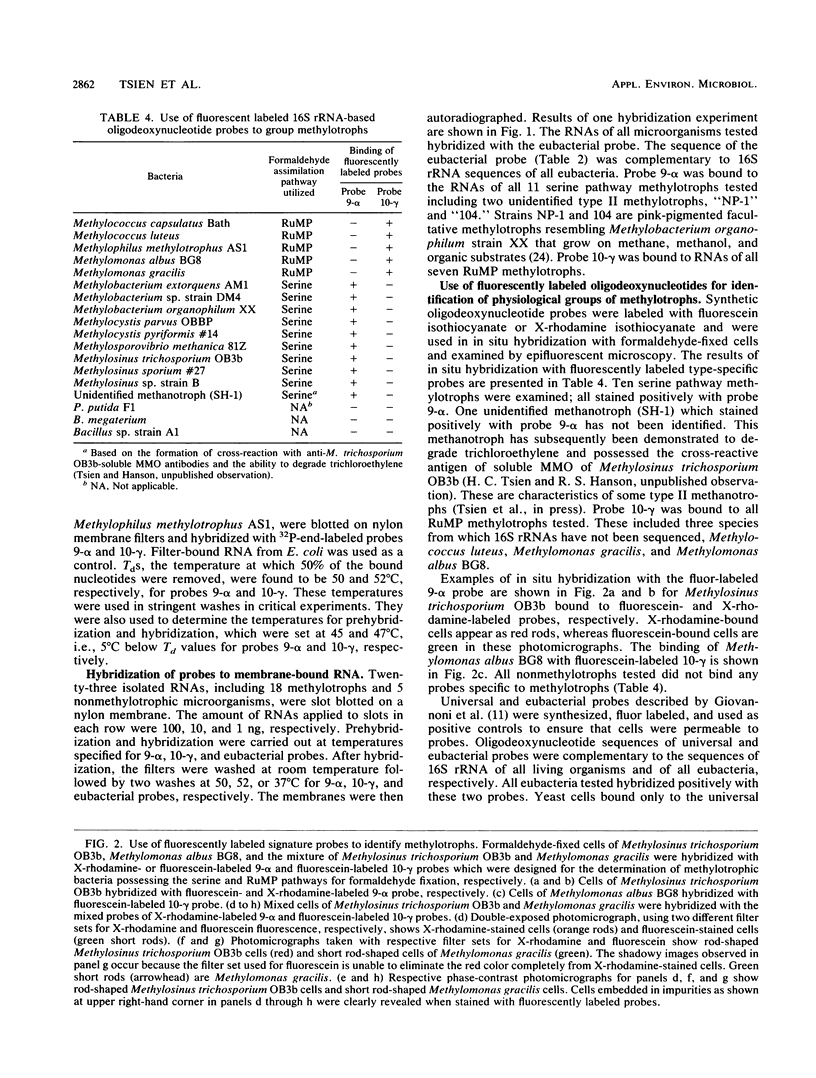
Oligodeoxynucleotide sequences that uniquely complemented 16S rRNAs of each group of methylotrophs were synthesized and used as hybridization probes for the identification of methylotrophic bacteria possessing the serine and ribulose monophosphate (RuMP) pathways for formaldehyde fixation. The specificity of the probes was determined by hybridizing radiolabeled probes with slot-blotted RNAs of methylotrophs and other eubacteria followed by autoradiography. The washing temperature was determined experimentally to be 50 and 52 degrees C for 9-alpha (serine pathway) and 10-gamma (RuMP pathway) probes, respectively. RNAs isolated from serine pathway methylotrophs bound to probe 9-alpha, and RNAs from RuMP pathway methylotrophs bound to probe 10-gamma. Nonmethylotrophic eubacterial RNAs did not bind to either probe. The probes were also labeled with fluorescent dyes. Cells fixed to microscope slides were hybridized with these probes, washed, and examined in a fluorescence microscope equipped with appropriate filter sets. Cells of methylotrophic bacteria possessing the serine or RuMP pathway specifically bind probes designed for each group. Samples with a mixture of cells of type I and II methanotrophs were detected and differentiated with single probes or mixed probes labeled with different fluorescent dyes, which enabled the detection of both types of cells in the same microscopic field.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amann R. I., Krumholz L., Stahl D. A. Fluorescent-oligonucleotide probing of whole cells for determinative, phylogenetic, and environmental studies in microbiology. J Bacteriol. 1990 Feb;172(2):762–770. doi: 10.1128/jb.172.2.762-770.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong E. F., Wickham G. S., Pace N. R. Phylogenetic stains: ribosomal RNA-based probes for the identification of single cells. Science. 1989 Mar 10;243(4896):1360–1363. doi: 10.1126/science.2466341. [DOI] [PubMed] [Google Scholar]
- Fox G. E., Stackebrandt E., Hespell R. B., Gibson J., Maniloff J., Dyer T. A., Wolfe R. S., Balch W. E., Tanner R. S., Magrum L. J. The phylogeny of prokaryotes. Science. 1980 Jul 25;209(4455):457–463. doi: 10.1126/science.6771870. [DOI] [PubMed] [Google Scholar]
- Giovannoni S. J., DeLong E. F., Olsen G. J., Pace N. R. Phylogenetic group-specific oligodeoxynucleotide probes for identification of single microbial cells. J Bacteriol. 1988 Feb;170(2):720–726. doi: 10.1128/jb.170.2.720-726.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins I. J., Best D. J., Hammond R. C. New findings in methane-utilizing bacteria highlight their importance in the biosphere and their commercial potential. Nature. 1980 Aug 7;286(5773):561–564. doi: 10.1038/286561a0. [DOI] [PubMed] [Google Scholar]
- Higgins I. J., Hammond R. C., Sariaslani F. S., Best D., Davies M. M., Tryhorn S. E., Taylor F. Biotransformation of hydrocarbons and related compounds by whole organism suspensions of methane-grown methylosinus trichosporium OB 3b. Biochem Biophys Res Commun. 1979 Jul 27;89(2):671–677. doi: 10.1016/0006-291x(79)90682-x. [DOI] [PubMed] [Google Scholar]
- Holben William E., Jansson Janet K., Chelm Barry K., Tiedje James M. DNA Probe Method for the Detection of Specific Microorganisms in the Soil Bacterial Community. Appl Environ Microbiol. 1988 Mar;54(3):703–711. doi: 10.1128/aem.54.3.703-711.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. D., Nogueira Araujo G. M. A simple method of reducing the fading of immunofluorescence during microscopy. J Immunol Methods. 1981;43(3):349–350. doi: 10.1016/0022-1759(81)90183-6. [DOI] [PubMed] [Google Scholar]
- Maclennan D. G., Ousby J. C., Vasey R. B., Cotton N. T. The influence of dissolved oxygen on Pseudomonas AM1 grown on methanol in continuous culture. J Gen Microbiol. 1971 Dec;69(3):395–404. doi: 10.1099/00221287-69-3-395. [DOI] [PubMed] [Google Scholar]
- Miller R. E., Guengerich F. P. Metabolism of trichloroethylene in isolated hepatocytes, microsomes, and reconstituted enzyme systems containing cytochrome P-450. Cancer Res. 1983 Mar;43(3):1145–1152. [PubMed] [Google Scholar]
- Olsen G. J., Lane D. J., Giovannoni S. J., Pace N. R., Stahl D. A. Microbial ecology and evolution: a ribosomal RNA approach. Annu Rev Microbiol. 1986;40:337–365. doi: 10.1146/annurev.mi.40.100186.002005. [DOI] [PubMed] [Google Scholar]
- Patt T. E., Cole G. C., Bland J., Hanson R. S. Isolation and characterization of bacteria that grow on methane and organic compounds as sole sources of carbon and energy. J Bacteriol. 1974 Nov;120(2):955–964. doi: 10.1128/jb.120.2.955-964.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pütz J., Meinert F., Wyss U., Ehlers R. U., Stackebrandt E. Development and application of oligonucleotide probes for molecular identification of Xenorhabdus species. Appl Environ Microbiol. 1990 Jan;56(1):181–186. doi: 10.1128/aem.56.1.181-186.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed W. M., Dugan P. R. Distribution of Methylomonas methanica and Methylosinus trichosporium in Cleveland Harbor as Determined by an Indirect Fluorescent Antibody-Membrane Filter Technique. Appl Environ Microbiol. 1978 Feb;35(2):422–430. doi: 10.1128/aem.35.2.422-430.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl D. A., Flesher B., Mansfield H. R., Montgomery L. Use of phylogenetically based hybridization probes for studies of ruminal microbial ecology. Appl Environ Microbiol. 1988 May;54(5):1079–1084. doi: 10.1128/aem.54.5.1079-1084.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling D. I., Dalton H. Properties of the methane mono-oxygenase from extracts of Methylosinus trichosporium OB3b and evidence for its similarity to the enzyme from Methylococcus capsulatus (Bath). Eur J Biochem. 1979 May 2;96(1):205–212. doi: 10.1111/j.1432-1033.1979.tb13030.x. [DOI] [PubMed] [Google Scholar]
- Tsien H. C., Brusseau G. A., Hanson R. S., Waclett L. P. Biodegradation of trichloroethylene by Methylosinus trichosporium OB3b. Appl Environ Microbiol. 1989 Dec;55(12):3155–3161. doi: 10.1128/aem.55.12.3155-3161.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji K., Tsien H. C., Hanson R. S., DePalma S. R., Scholtz R., LaRoche S. 16S ribosomal RNA sequence analysis for determination of phylogenetic relationship among methylotrophs. J Gen Microbiol. 1990 Jan;136(1):1–10. doi: 10.1099/00221287-136-1-1. [DOI] [PubMed] [Google Scholar]
- Wackett L. P., Gibson D. T. Degradation of trichloroethylene by toluene dioxygenase in whole-cell studies with Pseudomonas putida F1. Appl Environ Microbiol. 1988 Jul;54(7):1703–1708. doi: 10.1128/aem.54.7.1703-1708.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windass J. D., Worsey M. J., Pioli E. M., Pioli D., Barth P. T., Atherton K. T., Dart E. C., Byrom D., Powell K., Senior P. J. Improved conversion of methanol to single-cell protein by Methylophilus methylotrophus. Nature. 1980 Oct 2;287(5781):396–401. doi: 10.1038/287396a0. [DOI] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]




