Abstract
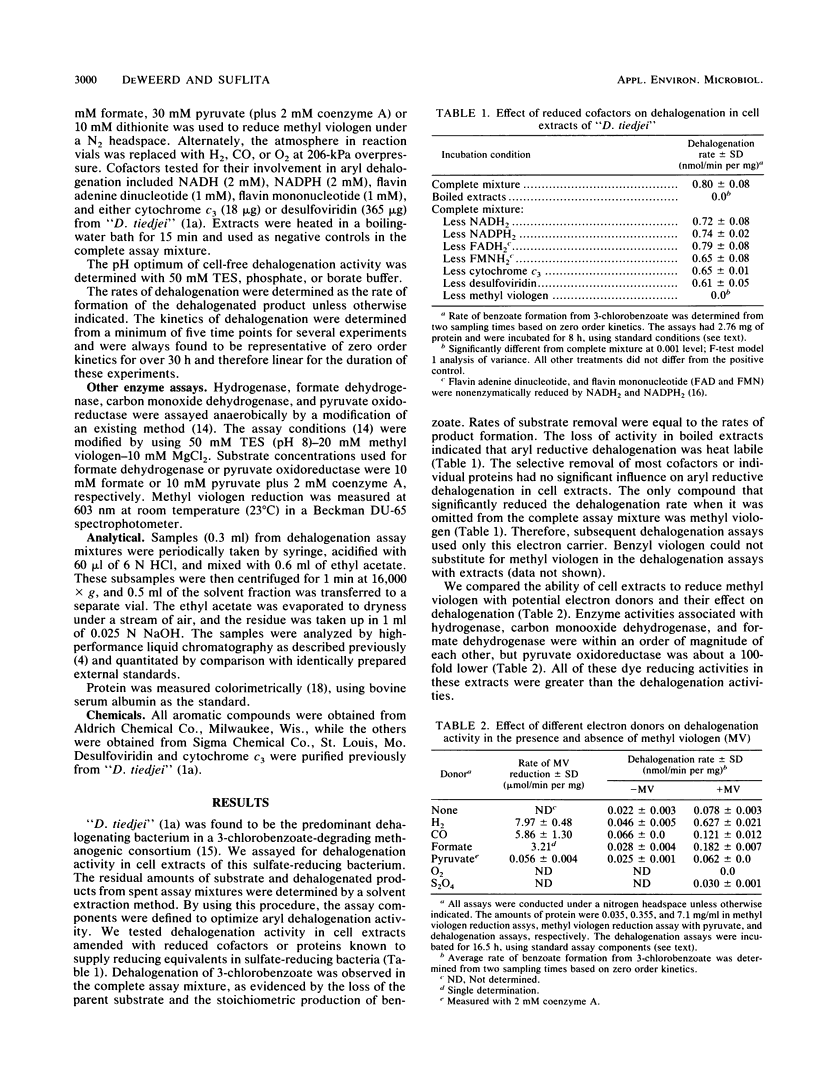
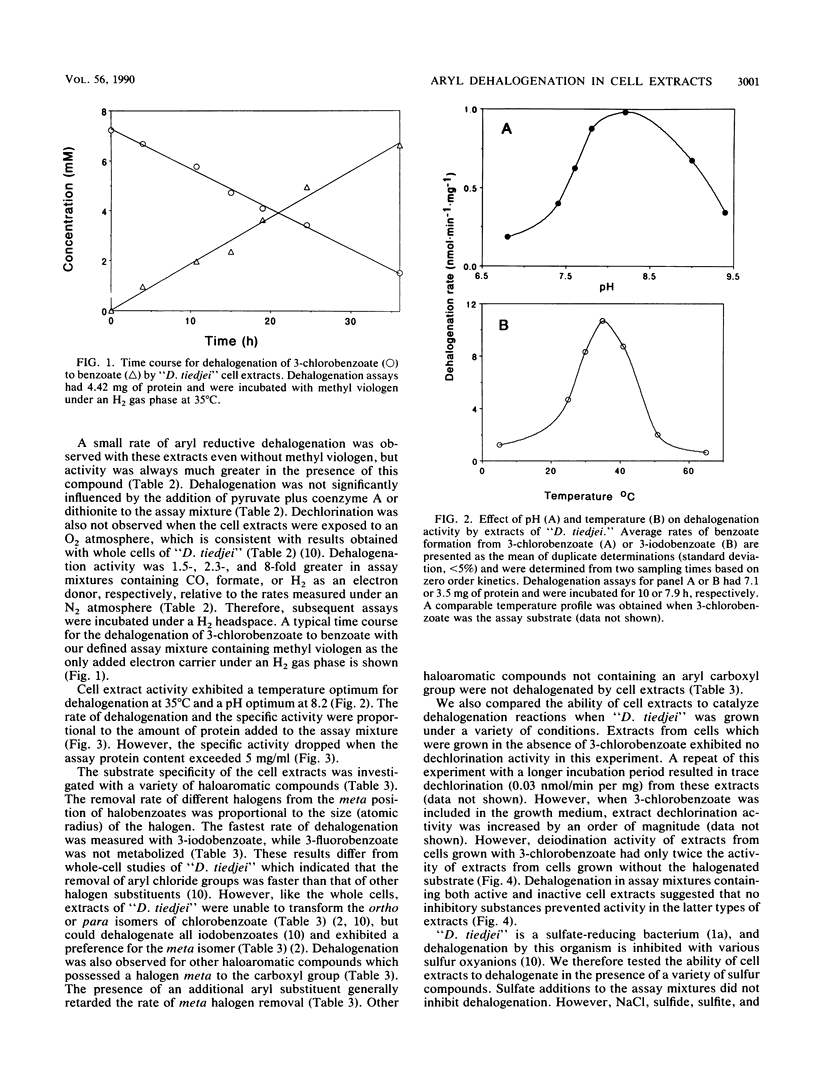
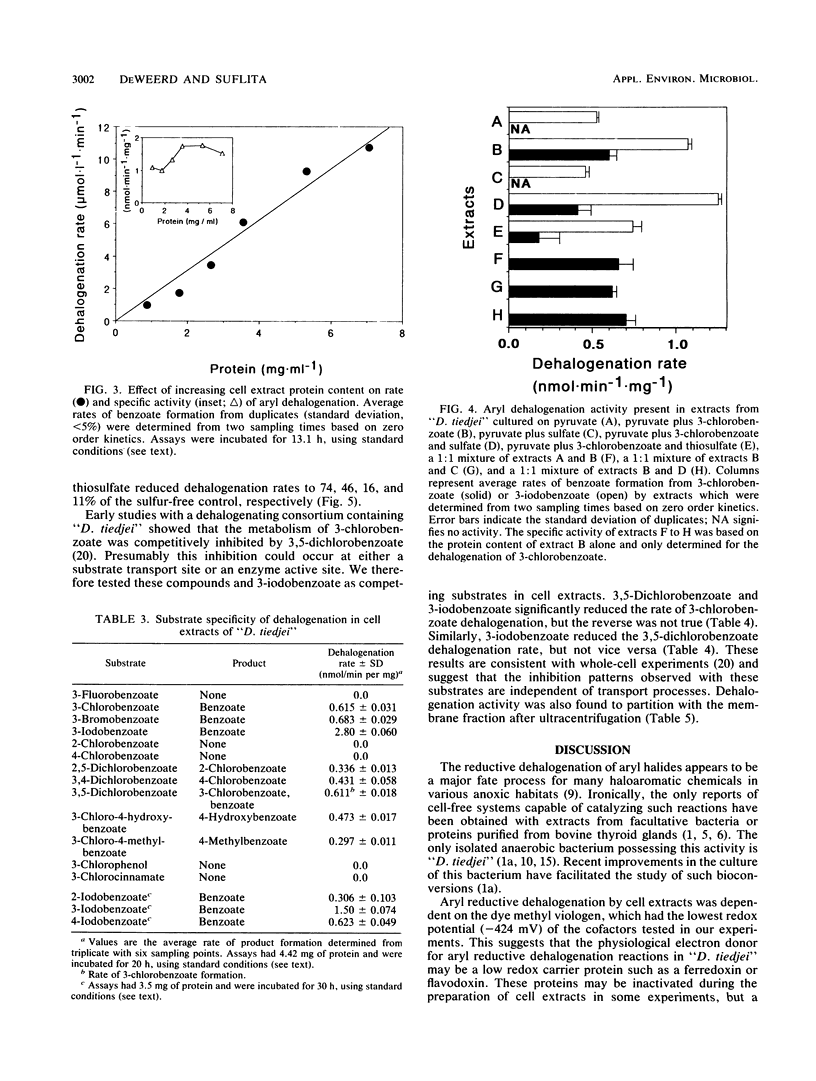
We studied the transformation of halogenated benzoates by cell extracts of a dehalogenating anaerobe, “Desulfomonile tiedjei.” We found that cell extracts possessed aryl reductive dehalogenation activity. The activity was heat labile and dependent on the addition of reduced methyl viologen, but not on that of reduced NAD, NADP, flavin mononucleotide, flavin adenine dinucleotide, desulfoviridin, cytochrome c3, or benzyl viologen. Dehalogenation activity in extracts was stimulated by formate, CO, or H2, but not by pyruvate plus coenzyme A or by dithionite. The pH and temperature optima for aryl dehalogenation were 8.2 and 35°C, respectively. The rate of dehalogenation was proportional to the amount of protein in the assay mixture. The substrate specificity of aryl dehalogenation activity for various aromatic compounds in “D. tiedjei” cell extracts was identical to that of whole cells, except differences were observed in the relative rates of halobenzoate transformation. Dehalogenation was 10-fold greater in “D. tiedjei” extracts prepared from cells cultured in the presence of 3-chlorobenzoate, suggesting that the activity was inducible. Aryl reductive dehalogenation in extracts was inhibited by sulfite, sulfide, and thiosulfate, but not sulfate. Experiments with combinations of substrates suggested that cell extracts dehalogenated 3-iodobenzoate more readily than either 3,5-dichlorobenzoate or 3-chlorobenzoate. Dehalogenation activity was found to be membrane associated. This is the first report characterizing aryl dehalogenation activity in cell extracts of an obligate anaerobe.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apajalahti J. H., Salkinoja-Salonen M. S. Complete dechlorination of tetrachlorohydroquinone by cell extracts of pentachlorophenol-induced Rhodococcus chlorophenolicus. J Bacteriol. 1987 Nov;169(11):5125–5130. doi: 10.1128/jb.169.11.5125-5130.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson S. A., Suflita J. M. Extrapolation of biodegradation results to groundwater aquifers: reductive dehalogenation of aromatic compounds. Appl Environ Microbiol. 1986 Oct;52(4):681–688. doi: 10.1128/aem.52.4.681-688.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami A., Rosenberg I. N. Characterization of a flavoprotein iodotyrosine deiodinase from bovine thyroid. Flavin nucleotide binding and oxidation-reduction properties. J Biol Chem. 1979 Dec 25;254(24):12326–12330. [PubMed] [Google Scholar]
- Häggblom M. M., Janke D., Salkinoja-Salonen M. S. Hydroxylation and Dechlorination of Tetrachlorohydroquinone by Rhodococcus sp. Strain CP-2 Cell Extracts. Appl Environ Microbiol. 1989 Feb;55(2):516–519. doi: 10.1128/aem.55.2.516-519.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkfield T. G., Tiedje J. M. Characterization of the requirements and substrates for reductive dehalogenation by strain DCB-1. J Ind Microbiol. 1990 Jan;5(1):9–15. doi: 10.1007/BF01569601. [DOI] [PubMed] [Google Scholar]
- Nastainczyk W., Ahr H. J., Ullrich V. The reductive metabolism of halogenated alkanes by liver microsomal cytochrome P450. Biochem Pharmacol. 1982 Feb 1;31(3):391–396. doi: 10.1016/0006-2952(82)90187-3. [DOI] [PubMed] [Google Scholar]
- Shelton D. R., Tiedje J. M. Isolation and partial characterization of bacteria in an anaerobic consortium that mineralizes 3-chlorobenzoic Acid. Appl Environ Microbiol. 1984 Oct;48(4):840–848. doi: 10.1128/aem.48.4.840-848.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Stevens T. O., Linkfield T. G., Tiedje J. M. Physiological characterization of strain DCB-1, a unique dehalogenating sulfidogenic bacterium. Appl Environ Microbiol. 1988 Dec;54(12):2938–2943. doi: 10.1128/aem.54.12.2938-2943.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suflita J. M., Horowitz A., Shelton D. R., Tiedje J. M. Dehalogenation: a novel pathway for the anaerobic biodegradation of haloaromatic compounds. Science. 1982 Dec 10;218(4577):1115–1117. doi: 10.1126/science.218.4577.1115. [DOI] [PubMed] [Google Scholar]
- Suflita J. M., Robinson J. A., Tiedje J. M. Kinetics of microbial dehalogenation of haloaromatic substrates in methanogenic environments. Appl Environ Microbiol. 1983 May;45(5):1466–1473. doi: 10.1128/aem.45.5.1466-1473.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]


