Abstract
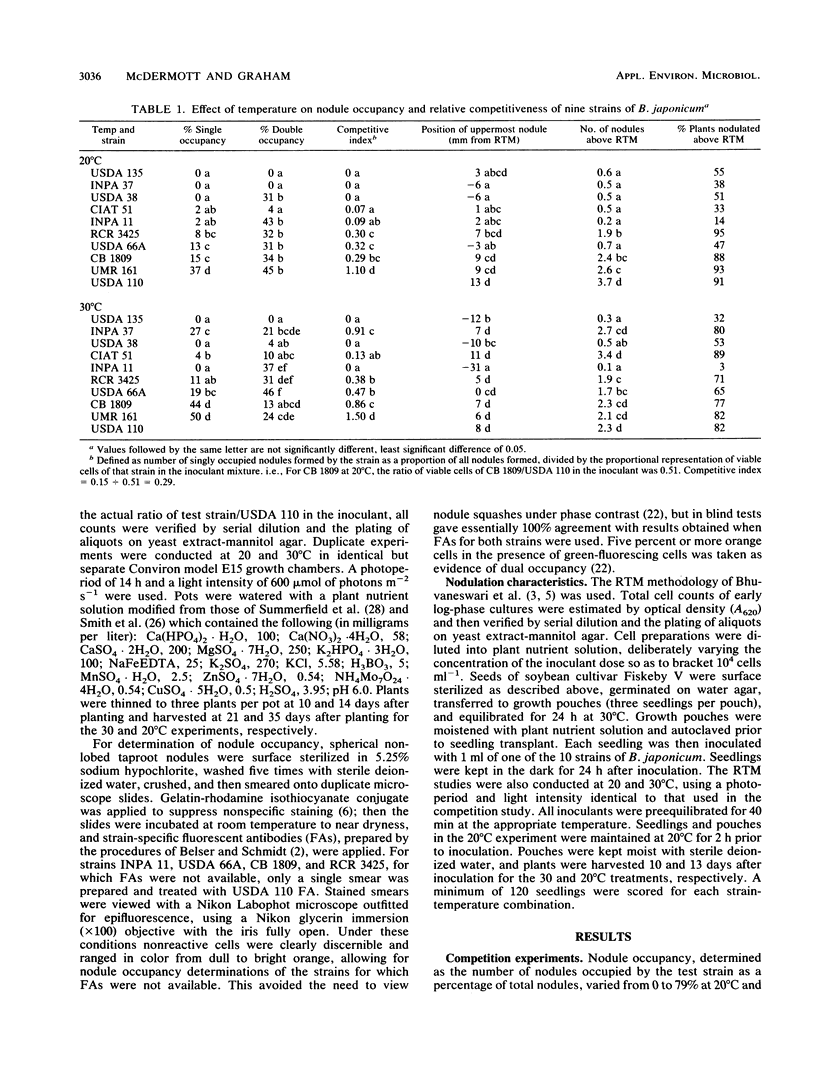
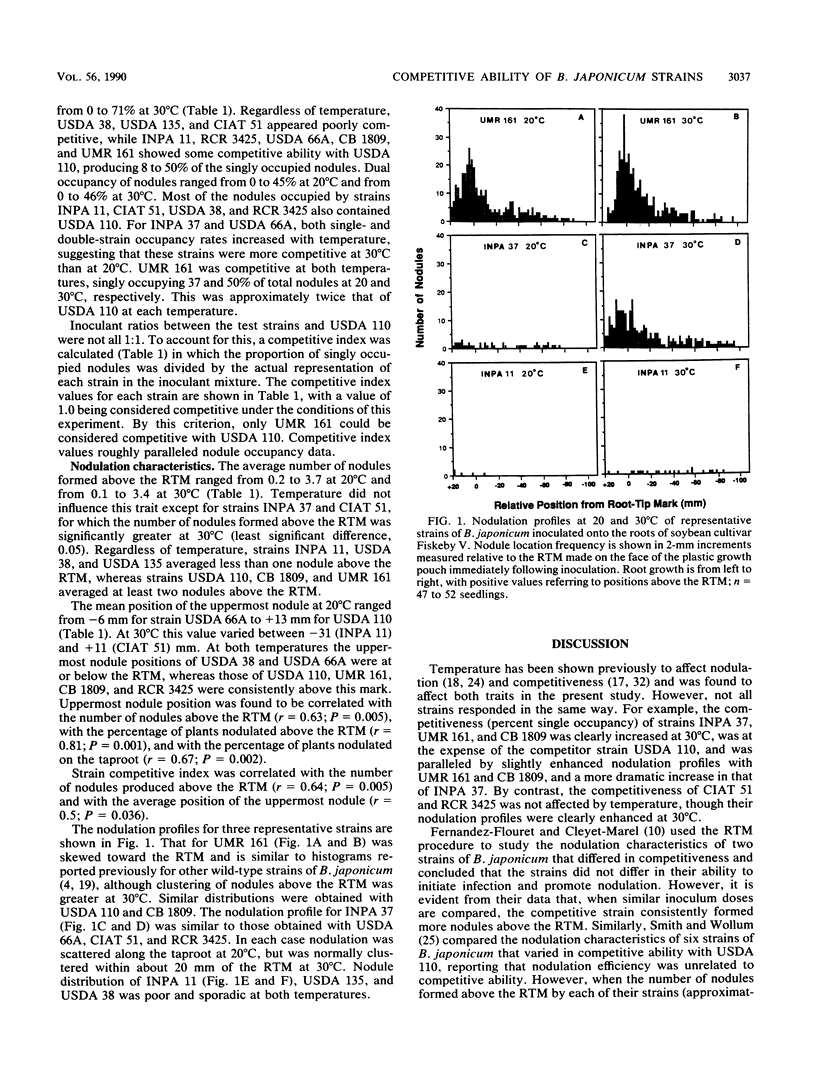
In the American Midwest, superior N2-fixing inoculant strains of Bradyrhizobium japonicum consistently fail to produce the majority of nodules on the roots of field-grown soybean. Poor nodulation by inoculant strains is partly due to their inability to stay abreast of the expanding soybean root system in numbers sufficient for them to be competitive with indigenous bradyrhizobia. However, certain strains are noncompetitive even when numerical dominance is not a factor. In this study, we tested the hypothesis that the nodule occupancy achieved by strains is related to their nodule-forming efficiency. The nodulation characteristics and competitiveness of nine strains of B. japonicum were compared at both 20 and 30°C. The root tip marking technique was used, with the nodule-forming efficiency of each strain estimated from the average position of the uppermost nodule and the number of nodules formed above the root tip mark. The competitiveness of the nine strains relative to B. japonicum USDA 110 was determined by using immunofluorescence to identify nodule occupants. The strains differed significantly in competitiveness with USDA 110 and in nodulation characteristics, strains that were poor competitors usually proving to be inferior in both the average position of the uppermost root nodule and the number of nodules formed above the root tip mark. Thus, competitiveness was correlated with both the average position of the uppermost nodule (r = 0.5; P = 0.036) and the number of nodules formed above the root tip mark (r = 0.64; P = 0.005), while the position of the uppermost nodule was also correlated to the percentage of plants nodulated above the root tip mark (r = 0.81; P < 0.001) and the percentage of plants nodulated on the taproot (r = 0.67; P = 0.002).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amarger N., Lobreau J. P. Quantitative study of nodulation competitiveness in Rhizobium strains. Appl Environ Microbiol. 1982 Sep;44(3):583–588. doi: 10.1128/aem.44.3.583-588.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belser L. W., Schmidt E. L. Serological diversity within a terrestrial ammonia-oxidizing population. Appl Environ Microbiol. 1978 Oct;36(4):589–593. doi: 10.1128/aem.36.4.589-593.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuvaneswari T. V., Bhagwat A. A., Bauer W. D. Transient susceptibility of root cells in four common legumes to nodulation by rhizobia. Plant Physiol. 1981 Nov;68(5):1144–1149. doi: 10.1104/pp.68.5.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuvaneswari T. V., Lesniak A. P., Bauer W. D. Efficiency of nodule initiation in cowpea and soybean. Plant Physiol. 1988 Apr;86(4):1210–1215. doi: 10.1104/pp.86.4.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuvaneswari T. V., Turgeon B. G., Bauer W. D. Early Events in the Infection of Soybean (Glycine max L. Merr) by Rhizobium japonicum: I. LOCALIZATION OF INFECTIBLE ROOT CELLS. Plant Physiol. 1980 Dec;66(6):1027–1031. doi: 10.1104/pp.66.6.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlool B. B., Schmidt E. L. Nonspecific staining: its control in immunofluorescence examination of soil. Science. 1968 Nov 29;162(3857):1012–1014. doi: 10.1126/science.162.3857.1012. [DOI] [PubMed] [Google Scholar]
- Demezas D. H., Bottomley P. J. Interstrain Competition between Representatives of Indigenous Serotypes of Rhizobium trifolii. Appl Environ Microbiol. 1986 Nov;52(5):1020–1025. doi: 10.1128/aem.52.5.1020-1025.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling D. N., Broughton W. J. Competition for nodulation of legumes. Annu Rev Microbiol. 1986;40:131–157. doi: 10.1146/annurev.mi.40.100186.001023. [DOI] [PubMed] [Google Scholar]
- Handelsman J., Ugalde R. A., Brill W. J. Rhizobium meliloti competitiveness and the alfalfa agglutinin. J Bacteriol. 1984 Mar;157(3):703–707. doi: 10.1128/jb.157.3.703-707.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik N. S., Bauer W. D. When does the self-regulatory response elicited in soybean root after inoculation occur? Plant Physiol. 1988 Nov;88(3):537–539. doi: 10.1104/pp.88.3.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott T. R., Graham P. H. Bradyrhizobium japonicum Inoculant Mobility, Nodule Occupancy, and Acetylene Reduction in the Soybean Root System. Appl Environ Microbiol. 1989 Oct;55(10):2493–2498. doi: 10.1128/aem.55.10.2493-2498.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moawad H. A., Ellis W. R., Schmidt E. L. Rhizosphere Response as a Factor in Competition Among Three Serogroups of Indigenous Rhizobium japonicum for Nodulation of Field-Grown Soybeans. Appl Environ Microbiol. 1984 Apr;47(4):607–612. doi: 10.1128/aem.47.4.607-612.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Geoffrey B., Wollum A. G. Nodulation of Glycine max by Six Bradyrhizobium japonicum Strains with Different Competitive Abilities. Appl Environ Microbiol. 1989 Aug;55(8):1957–1962. doi: 10.1128/aem.55.8.1957-1962.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadisirisuk P., Danso S. K., Hardarson G., Bowen G. D. Influence of Bradyrhizobium japonicum Location and Movement on Nodulation and Nitrogen Fixation in Soybeans. Appl Environ Microbiol. 1989 Jul;55(7):1711–1716. doi: 10.1128/aem.55.7.1711-1716.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]


