Abstract
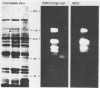
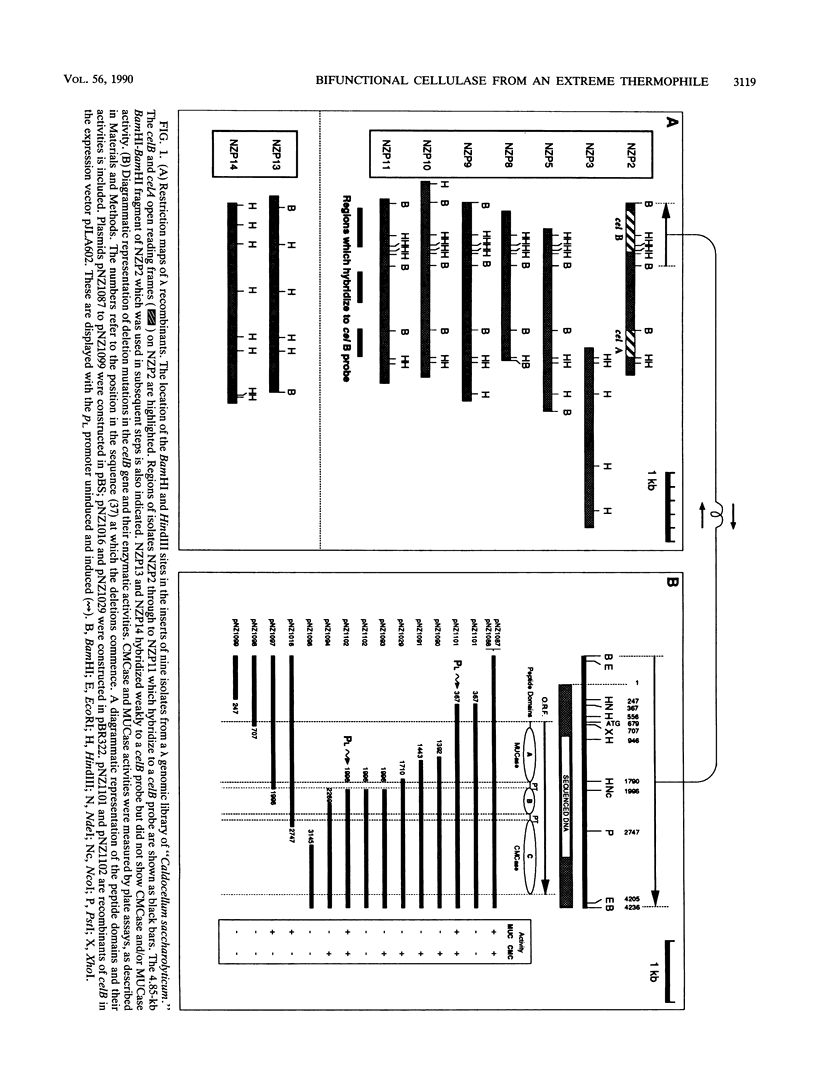
"Caldocellum saccharolyticum" is an obligatory anaerobic thermophilic bacterium. A gene from this organism, designated celB, has been cloned in Escherichia coli as part of a bacteriophage lambda gene library. This gene produces a thermostable cellulase that shows both endoglucanase and exoglucanase activities on test substrates and is able to degrade crystalline cellulose to glucose. The sequence of celB has homology with both exo- and endoglucanases described by others. It appears to have a central domain without enzymatic activity which is joined to the enzymatic domains by runs of amino acids rich in proline and threonine (PT boxes). Deletion analysis shows that the exoglucanase activity is located in the amino-terminal domain of the enzyme and that endoglucanase activity is located in the carboxy-terminal domain. There are internal transcriptional and translational start sites within the gene. The intact gene has been cloned into a temperature-inducible expression vector, pJLA602, and overexpressed in E. coli. Polyacrylamide gel electrophoresis showed that celB produced a protein with a molecular weight of 118,000 to 120,000. A number of smaller proteins with activity against carboxymethyl cellulose and 4-methyl umbelliferyl-beta-D-cellobioside were also produced. These are believed to be the result of alternative translational start sites and/or proteolytic degradation products of the translated gene product.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhikhabhai R., Johansson G., Pettersson G. Isolation of cellulolytic enzymes from Trichoderma reesei QM 9414. J Appl Biochem. 1984 Oct-Dec;6(5-6):336–345. [PubMed] [Google Scholar]
- Béguin P., Cornet P., Aubert J. P. Sequence of a cellulase gene of the thermophilic bacterium Clostridium thermocellum. J Bacteriol. 1985 Apr;162(1):102–105. doi: 10.1128/jb.162.1.102-105.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béguin P. Detection of cellulase activity in polyacrylamide gels using Congo red-stained agar replicas. Anal Biochem. 1983 Jun;131(2):333–336. doi: 10.1016/0003-2697(83)90178-1. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Chernoglazov V. M., Jafarova A. N., Klyosov A. A. Continuous photometric determination of endo-1,4-beta-D-glucanase (cellulase) activity using 4-methylumbelliferyl-beta-D-cellobioside as a substrate. Anal Biochem. 1989 May 15;179(1):186–189. doi: 10.1016/0003-2697(89)90222-4. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Dierstein R., Wickner W. Requirements for substrate recognition by bacterial leader peptidase. EMBO J. 1986 Feb;5(2):427–431. doi: 10.1002/j.1460-2075.1986.tb04228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Gilkes N. R., Warren R. A., Miller R. C., Jr, Kilburn D. G. Precise excision of the cellulose binding domains from two Cellulomonas fimi cellulases by a homologous protease and the effect on catalysis. J Biol Chem. 1988 Jul 25;263(21):10401–10407. [PubMed] [Google Scholar]
- Grépinet O., Béguin P. Sequence of the cellulase gene of Clostridium thermocellum coding for endoglucanase B. Nucleic Acids Res. 1986 Feb 25;14(4):1791–1799. doi: 10.1093/nar/14.4.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiseppi A., Cami B., Aymeric J. L., Ball G., Creuzet N. Homology between endoglucanase Z of Erwinia chrysanthemi and endoglucanases of Bacillus subtilis and alkalophilic Bacillus. Mol Microbiol. 1988 Jan;2(1):159–164. doi: 10.1111/j.1365-2958.1988.tb00017.x. [DOI] [PubMed] [Google Scholar]
- Hall J., Hazlewood G. P., Barker P. J., Gilbert H. J. Conserved reiterated domains in Clostridium thermocellum endoglucanases are not essential for catalytic activity. Gene. 1988 Sep 15;69(1):29–38. doi: 10.1016/0378-1119(88)90375-7. [DOI] [PubMed] [Google Scholar]
- Joliff G., Béguin P., Aubert J. P. Nucleotide sequence of the cellulase gene celD encoding endoglucanase D of Clostridium thermocellum. Nucleic Acids Res. 1986 Nov 11;14(21):8605–8613. doi: 10.1093/nar/14.21.8605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karn J., Brenner S., Barnett L., Cesareni G. Novel bacteriophage lambda cloning vector. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5172–5176. doi: 10.1073/pnas.77.9.5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langsford M. L., Gilkes N. R., Singh B., Moser B., Miller R. C., Jr, Warren R. A., Kilburn D. G. Glycosylation of bacterial cellulases prevents proteolytic cleavage between functional domains. FEBS Lett. 1987 Dec 10;225(1-2):163–167. doi: 10.1016/0014-5793(87)81150-x. [DOI] [PubMed] [Google Scholar]
- Love D. R., Fisher R., Bergquist P. L. Sequence structure and expression of a cloned beta-glucosidase gene from an extreme thermophile. Mol Gen Genet. 1988 Jul;213(1):84–92. doi: 10.1007/BF00333402. [DOI] [PubMed] [Google Scholar]
- Lüthi E., Love D. R., McAnulty J., Wallace C., Caughey P. A., Saul D., Bergquist P. L. Cloning, sequence analysis, and expression of genes encoding xylan-degrading enzymes from the thermophile "Caldocellum saccharolyticum". Appl Environ Microbiol. 1990 Apr;56(4):1017–1024. doi: 10.1128/aem.56.4.1017-1024.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKay R. M., Lo A., Willick G., Zuker M., Baird S., Dove M., Moranelli F., Seligy V. Structure of a Bacillus subtilis endo-beta-1,4-glucanase gene. Nucleic Acids Res. 1986 Nov 25;14(22):9159–9170. doi: 10.1093/nar/14.22.9159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill G., Goh S. H., Warren R. A., Kilburn D. G., Miller R. C., Jr Structure of the gene encoding the exoglucanase of Cellulomonas fimi. Gene. 1986;44(2-3):325–330. doi: 10.1016/0378-1119(86)90197-6. [DOI] [PubMed] [Google Scholar]
- Rackwitz H. R., Zehetner G., Frischauf A. M., Lehrach H. Rapid restriction mapping of DNA cloned in lambda phage vectors. Gene. 1984 Oct;30(1-3):195–200. doi: 10.1016/0378-1119(84)90120-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saul D. J., Williams L. C., Love D. R., Chamley L. W., Bergquist P. L. Nucleotide sequence of a gene from Caldocellum saccharolyticum encoding for exocellulase and endocellulase activity. Nucleic Acids Res. 1989 Jan 11;17(1):439–439. doi: 10.1093/nar/17.1.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saul D., Spiers A. J., McAnulty J., Gibbs M. G., Bergquist P. L., Hill D. F. Nucleotide sequence and replication characteristics of RepFIB, a basic replicon of IncF plasmids. J Bacteriol. 1989 May;171(5):2697–2707. doi: 10.1128/jb.171.5.2697-2707.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauder B., Blöcker H., Frank R., McCarthy J. E. Inducible expression vectors incorporating the Escherichia coli atpE translational initiation region. Gene. 1987;52(2-3):279–283. doi: 10.1016/0378-1119(87)90054-0. [DOI] [PubMed] [Google Scholar]
- Schwarz W. H., Gräbnitz F., Staudenbauer W. L. Properties of a Clostridium thermocellum Endoglucanase Produced in Escherichia coli. Appl Environ Microbiol. 1986 Jun;51(6):1293–1299. doi: 10.1128/aem.51.6.1293-1299.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short J. M., Fernandez J. M., Sorge J. A., Huse W. D. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 1988 Aug 11;16(15):7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren R. A., Gerhard B., Gilkes N. R., Owolabi J. B., Kilburn D. G., Miller R. C., Jr A bifunctional exoglucanase-endoglucanase fusion protein. Gene. 1987;61(3):421–427. doi: 10.1016/0378-1119(87)90204-6. [DOI] [PubMed] [Google Scholar]
- West C. A., Elzanowski A., Yeh L. S., Barker W. C. Homologues of catalytic domains of Cellulomonas glucanases found in fungal and Bacillus glycosidases. FEMS Microbiol Lett. 1989 May;50(1-2):167–172. doi: 10.1016/0378-1097(89)90479-5. [DOI] [PubMed] [Google Scholar]
- Wong W. K., Gerhard B., Guo Z. M., Kilburn D. G., Warren A. J., Miller R. C., Jr Characterization and structure of an endoglucanase gene cenA of Cellulomonas fimi. Gene. 1986;44(2-3):315–324. doi: 10.1016/0378-1119(86)90196-4. [DOI] [PubMed] [Google Scholar]