Abstract
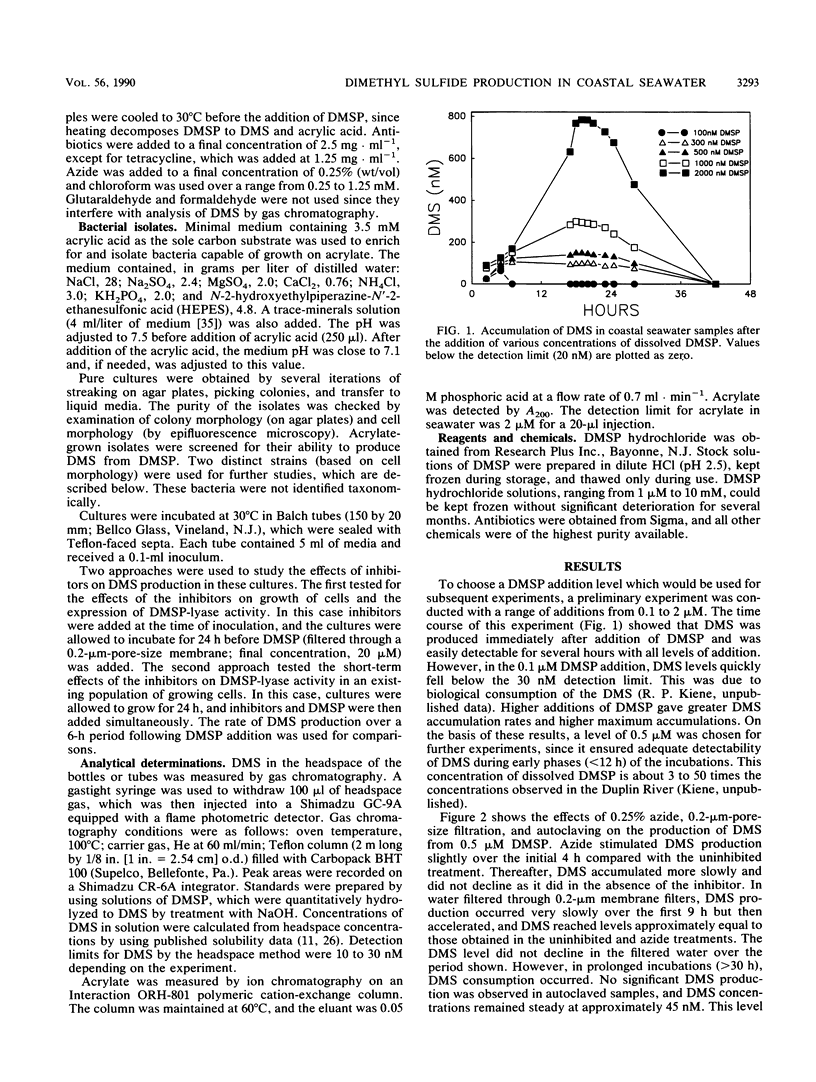
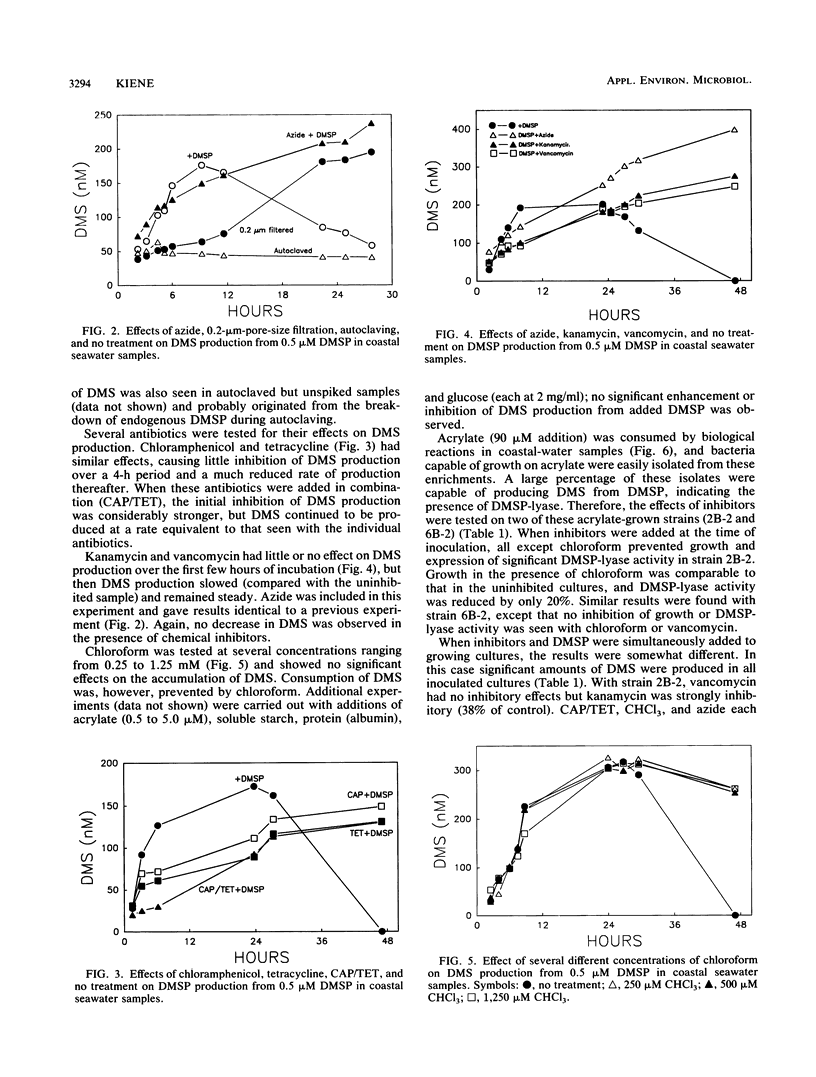
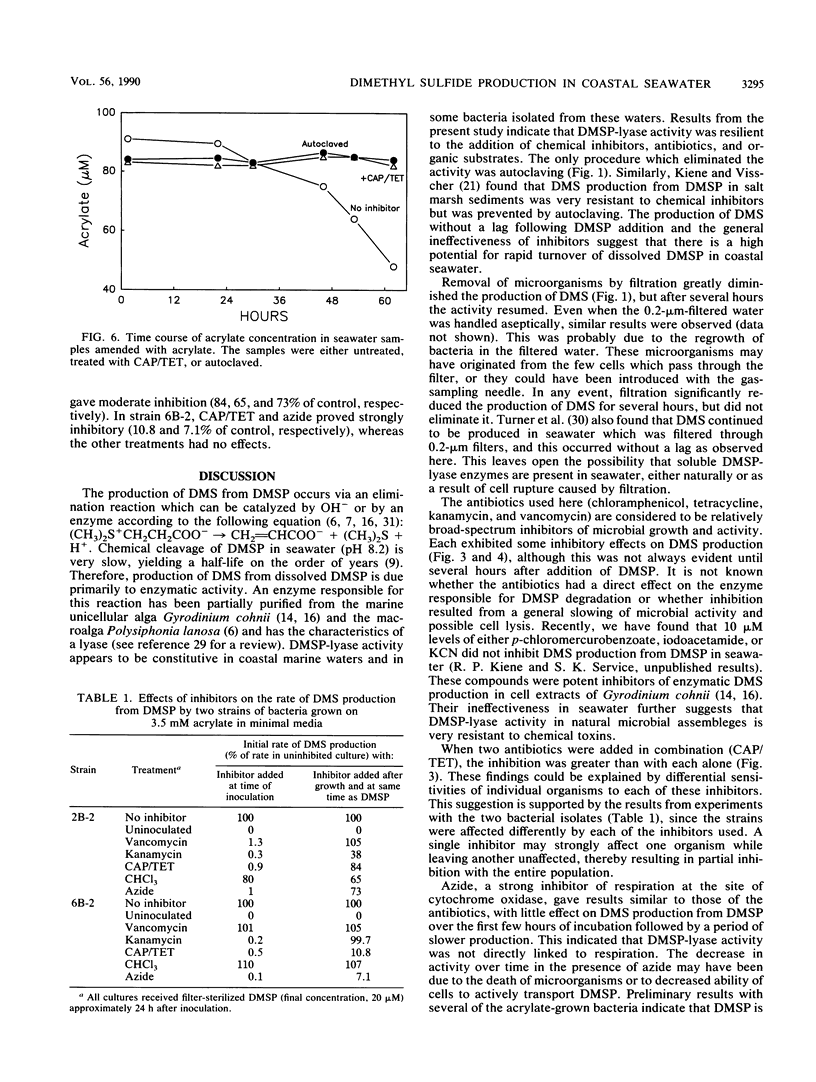
Dimethyl sulfide (DMS) was produced immediately after the addition of 0.1 to 2 μM β-dimethylsulfonio-propionate (DMSP) to coastal seawater samples. Azide had little effect on the initial rate of DMS production from 0.5 μM added DMSP, but decreased the rate of production after 6 h. Filtration of water samples through membrane filters (pore size, 0.2 μm) greatly reduced DMS production for approximately 10 h, after which time DMS production resumed at a high rate. Autoclaving completely eliminated the production of DMS. The antibiotics chloramphenicol, tetracycline, kanamycin, and vancomycin all had little effect on the accumulation of DMS over the first few hours of incubation, but produced significant inhibition thereafter. The effects of individual antibiotics were additive. Chloroform over a range of concentrations (0.25 to 1.25 mM) had no effects on DMS production. Similarly, organic amendments, including acrylate, glucose, protein, and starch, did not affect DMS accumulation from DMSP. Acrylate, a product of the enzymatic cleavage of DMSP, was metabolized in seawater samples, and two strains of bacteria were isolated with this compound as the growth substrate. These bacteria produced DMS from DMSP. The sensitivity to inhibitors with respect to growth and DMSP-lyase activity varied from strain to strain. These results illustrate the significant potential for microbial conversion of dissolved DMSP to DMS in coastal seawater.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON D. G., CANTONI G. L. Enzymatic cleavage of dimethylpropiothetin by Polysiphonia lanosa. J Biol Chem. 1956 Sep;222(1):171–177. [PubMed] [Google Scholar]
- Bauchop T. Inhibition of rumen methanogenesis by methane analogues. J Bacteriol. 1967 Jul;94(1):171–175. doi: 10.1128/jb.94.1.171-175.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacey J. W., Wakeham S. G. Oceanic dimethylsulfide: production during zooplankton grazing on phytoplankton. Science. 1986 Sep 19;233(4770):1314–1316. doi: 10.1126/science.233.4770.1314. [DOI] [PubMed] [Google Scholar]
- Kadota H., Ishida Y. Production of volatile sulfur compounds by microorganisms. Annu Rev Microbiol. 1972;26:127–138. doi: 10.1146/annurev.mi.26.100172.001015. [DOI] [PubMed] [Google Scholar]
- Kiene R. P., Visscher P. T. Production and fate of methylated sulfur compounds from methionine and dimethylsulfoniopropionate in anoxic salt marsh sediments. Appl Environ Microbiol. 1987 Oct;53(10):2426–2434. doi: 10.1128/aem.53.10.2426-2434.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nriagu J. O., Holdway D. A., Coker R. D. Biogenic sulfur and the acidity of rainfall in remote areas of Canada. Science. 1987 Sep 4;237(4819):1189–1192. doi: 10.1126/science.237.4819.1189. [DOI] [PubMed] [Google Scholar]
- Perroud B., Le Rudulier D. Glycine betaine transport in Escherichia coli: osmotic modulation. J Bacteriol. 1985 Jan;161(1):393–401. doi: 10.1128/jb.161.1.393-401.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieburth J. M. ANTIBIOTIC PROPERTIES OF ACRYLIC ACID, A FACTOR IN THE GASTROINTESTINAL ANTIBIOSIS OF POLAR MARINE ANIMALS. J Bacteriol. 1961 Jul;82(1):72–79. doi: 10.1128/jb.82.1.72-79.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAGNER C., STADTMAN E. R. Bacterial fermentation of dimethyl-beta-propiothetin. Arch Biochem Biophys. 1962 Aug;98:331–336. doi: 10.1016/0003-9861(62)90191-1. [DOI] [PubMed] [Google Scholar]


