Abstract
The lack of efficient transformation methods for aflatoxigenic Aspergillus parasiticus has been a major constraint for the study of aflatoxin biosynthesis at the genetic level. A transformation system with efficiencies of 30 to 50 stable transformants per microgram of DNA was developed for A. parasiticus by using the homologous pyrG gene. The pyrG gene from A. parasiticus was isolated by in situ plaque hybridization of a lambda genomic DNA library. Uridine auxotrophs of A. parasiticus ATCC 36537, a mutant blocked in aflatoxin biosynthesis, were isolated by selection on 5-fluoroorotic acid following nitrosoguanidine mutagenesis. Isolates with mutations in the pyrG gene resulting in elimination of orotidine monophosphate (OMP) decarboxylase activity were detected by assaying cell extracts for their ability to convert [14C]OMP to [14C]UMP. Transformation of A. parasiticus pyrG protoplasts with the homologous pyrG gene restored the fungal cells to prototrophy. Enzymatic analysis of cell extracts of transformant clones demonstrated that these extracts had the ability to convert [14C]OMP to [14C]UMP. Southern analysis of DNA purified from transformant clones indicated that both pUC19 vector sequences and pyrG sequences were integrated into the genome. The development of this pyrG transformation system should allow cloning of the aflatoxin-biosynthetic genes, which will be useful in studying the regulation of aflatoxin biosynthesis and may ultimately provide a means for controlling aflatoxin production in the field.
Full text
PDF

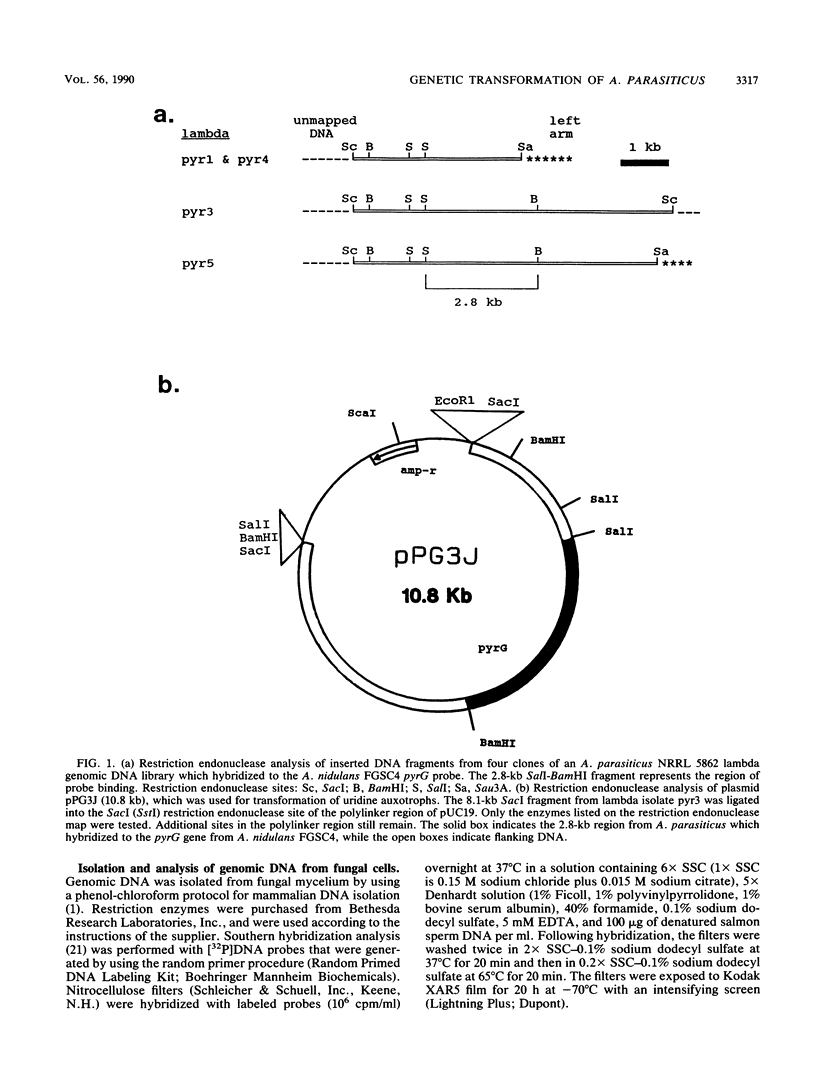
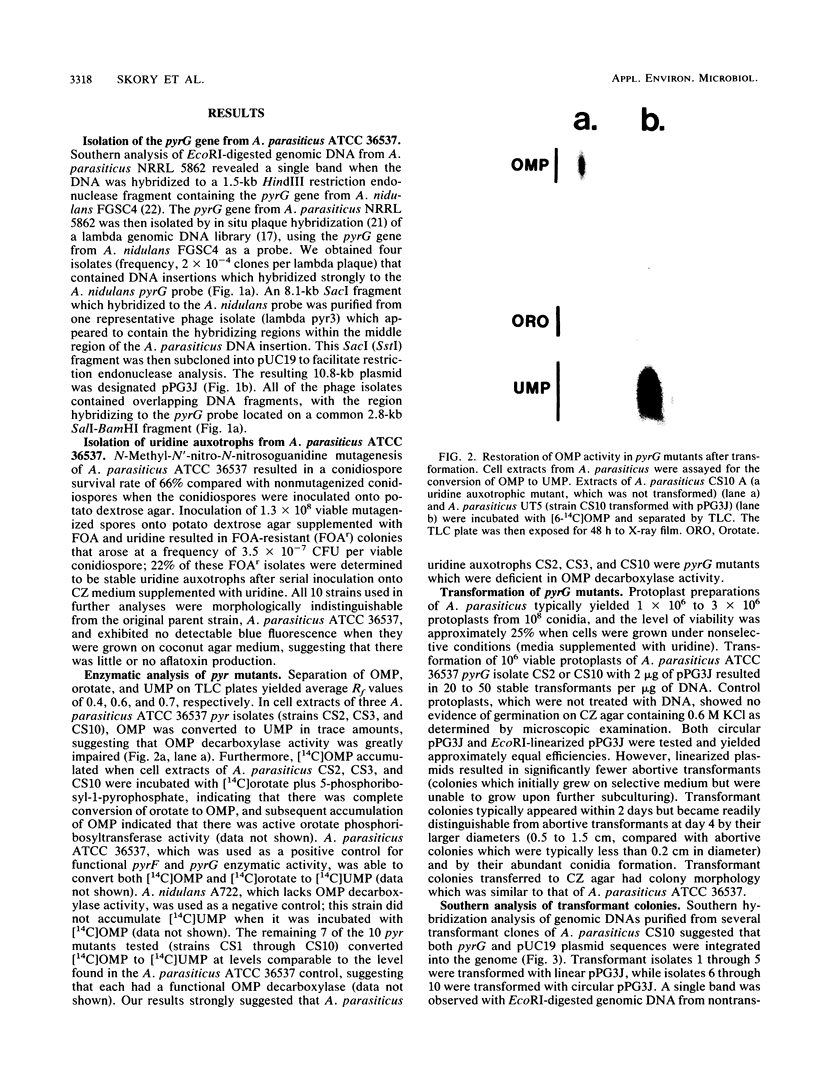


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett J. W., Lee L. S., Shoss S. M., Boudreaux G. H. Identification of averantin as an aflatoxin B1 precursor: placement in the biosynthetic pathway. Appl Environ Microbiol. 1980 Apr;39(4):835–839. doi: 10.1128/aem.39.4.835-839.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry C. L. The pathology of mycotoxins. J Pathol. 1988 Apr;154(4):301–311. doi: 10.1002/path.1711540405. [DOI] [PubMed] [Google Scholar]
- Bhatnagar D., Cleveland T. E., Lillehoj E. B. Enzymes in aflatoxin B1 biosynthesis: strategies for identifying pertinent genes. Mycopathologia. 1989 Sep;107(2-3):75–83. doi: 10.1007/BF00707542. [DOI] [PubMed] [Google Scholar]
- Boeke J. D., LaCroute F., Fink G. R. A positive selection for mutants lacking orotidine-5'-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol Gen Genet. 1984;197(2):345–346. doi: 10.1007/BF00330984. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cleveland T. E. Conversion of dihydro-O-methylsterigmatocystin to aflatoxin B2 by Aspergillus parasiticus. Arch Environ Contam Toxicol. 1989 May-Jun;18(3):429–433. doi: 10.1007/BF01062369. [DOI] [PubMed] [Google Scholar]
- Davis N. D., Iyer S. K., Diener U. L. Improved method of screening for aflatoxin with a coconut agar medium. Appl Environ Microbiol. 1987 Jul;53(7):1593–1595. doi: 10.1128/aem.53.7.1593-1595.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donkersloot J. A., Mateles R. I., Yang S. S. Isolation of averufin from a mutant of Aspergillus parasiticus impaired in aflatoxin biosynthesis. Biochem Biophys Res Commun. 1972 Jun 9;47(5):1051–1055. doi: 10.1016/0006-291x(72)90939-4. [DOI] [PubMed] [Google Scholar]
- Dorner J. W., Cole R. J., Diener U. L. The relationship of Aspergillus flavus and Aspergillus parasiticus with reference to production of aflatoxins and cyclopiazonic acid. Mycopathologia. 1984 Aug 30;87(1-2):13–15. doi: 10.1007/BF00436617. [DOI] [PubMed] [Google Scholar]
- Dutton M. F. Enzymes and aflatoxin biosynthesis. Microbiol Rev. 1988 Jun;52(2):274–295. doi: 10.1128/mr.52.2.274-295.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich K. Effect on aflatoxin production of competition between wild-type and mutant strains of Aspergillus parasiticus. Mycopathologia. 1987 Feb;97(2):93–96. doi: 10.1007/BF00436844. [DOI] [PubMed] [Google Scholar]
- Fincham J. R. Transformation in fungi. Microbiol Rev. 1989 Mar;53(1):148–170. doi: 10.1128/mr.53.1.148-170.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horng J. S., Linz J. E., Pestka J. J. Cloning and characterization of the trpC gene from an aflatoxigenic strain of Aspergillus parasiticus. Appl Environ Microbiol. 1989 Oct;55(10):2561–2568. doi: 10.1128/aem.55.10.2561-2568.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek C. F., Pohland A. E., Wood G. E. Worldwide occurrence of mycotoxins in foods and feeds--an update. J Assoc Off Anal Chem. 1989 Mar-Apr;72(2):223–230. [PubMed] [Google Scholar]
- Lee L. S., Bennett J. W., Cucullu A. F., Stanley J. B. Synthesis of versicolorin A by a mutant strain of Aspergillus parasiticus deficient in aflatoxin production. J Agric Food Chem. 1975 Nov-Dec;23(6):1132–1134. doi: 10.1021/jf60202a011. [DOI] [PubMed] [Google Scholar]
- Lee L., Bennett J. W., Goldblatt L. A., Lundin R. E. Norsolorinic acid from a mutant strain of Aspergillus parasiticus. J Am Oil Chem Soc. 1971 Feb;48(2):93–94. doi: 10.1007/BF02635696. [DOI] [PubMed] [Google Scholar]
- Oakley B. R., Rinehart J. E., Mitchell B. L., Oakley C. E., Carmona C., Gray G. L., May G. S. Cloning, mapping and molecular analysis of the pyrG (orotidine-5'-phosphate decarboxylase) gene of Aspergillus nidulans. Gene. 1987;61(3):385–399. doi: 10.1016/0378-1119(87)90201-0. [DOI] [PubMed] [Google Scholar]
- Payne G. A., Woloshuk C. P. Transformation of Aspergillus flavus to study aflatoxin biosynthesis. Mycopathologia. 1989 Sep;107(2-3):139–144. doi: 10.1007/BF00707551. [DOI] [PubMed] [Google Scholar]
- Stark A. A. Mutagenicity and carcinogenicity of mycotoxins: DNA binding as a possible mode of action. Annu Rev Microbiol. 1980;34:235–262. doi: 10.1146/annurev.mi.34.100180.001315. [DOI] [PubMed] [Google Scholar]
- Woloshuk C. P., Seip E. R., Payne G. A., Adkins C. R. Genetic transformation system for the aflatoxin-producing fungus Aspergillus flavus. Appl Environ Microbiol. 1989 Jan;55(1):86–90. doi: 10.1128/aem.55.1.86-90.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabe K., Ando Y., Hamasaki T. Biosynthetic relationship among aflatoxins B1, B2, G1, and G2. Appl Environ Microbiol. 1988 Aug;54(8):2101–2106. doi: 10.1128/aem.54.8.2101-2106.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]




