Abstract
The effects of a set of conditions on aminoglycoside uptake were determined. Membrane vesicles either with a membrane potential (delta psi) of -125 mV (adequate to drive lysine uptake) or with succinate, lactate, or phenazine methosulfate did not accumulate gentamicin unless components of protein synthesis were included. Ribosomally resistant (rpsL) Escherichia coli cells demonstrated energy-dependent phase II uptake similar to that of a streptomycin-susceptible strain of E. coli when treated with 100 micrograms of puromycin per ml. Puromycin (100 micrograms/ml) also increased the uptake of the cationic compounds polyamine and arginine. These studies support a role of protein synthesis in aminoglycoside uptake and in the development of energy-dependent phase II. delta psi of cells did not increase either at the initiation of or during energy-dependent phase II, showing that energy-dependent phase II is not due to an elevation of delta psi. In a Bacillus subtilis system, significant streptomycin uptake requires a threshold value of delta psi which varies depending upon the concentration of streptomycin used. At 25 micrograms/ml, the uptake of streptomycin reached maximal levels after exceeding the threshold value, whereas at 100 micrograms/ml there was a gradual increase of the uptake to the maximal after the threshold value was exceeded. Several studies supported the view that electron transport has a specific role other than its requirement to produce the cellular delta psi. The uptake of gentamicin was stimulated to a greater extent by phenazine methosulfate-ascorbate than by the ionophore nigericin in strains of E. coli, although nigericin stimulated delta psi to a greater degree. Cells with 25% of the normal quinone concentration had delta psi values identical to cells with the normal quinone concentration, but the quinone-deficient cells had a significantly lower rate of gentamicin uptake. KCN prevented gentamicin uptake but did not prevent the development of delta psi. The effects of ubiquinone depletion in an E. coli strain were more evident on gentamicin uptake than on ATP-driven glutamine transport or proton motive force-driven proline transport, consistent with a specific requirement for quinones in aminoglycoside uptake. A detailed explanation of the mechanism of accumulation of streptomycin and gentamicin and a proposed mechanism for killing bacterial cells by these agents have been provided.
Full text
PDF


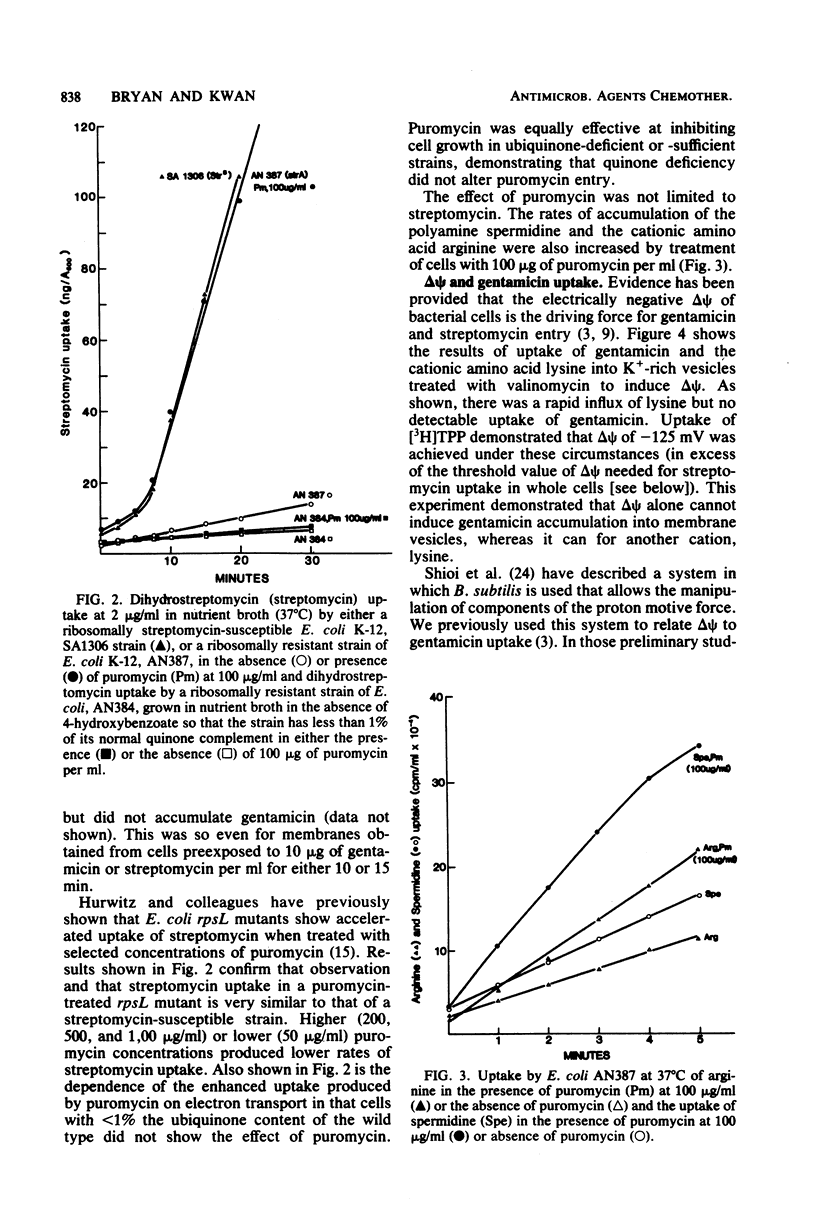
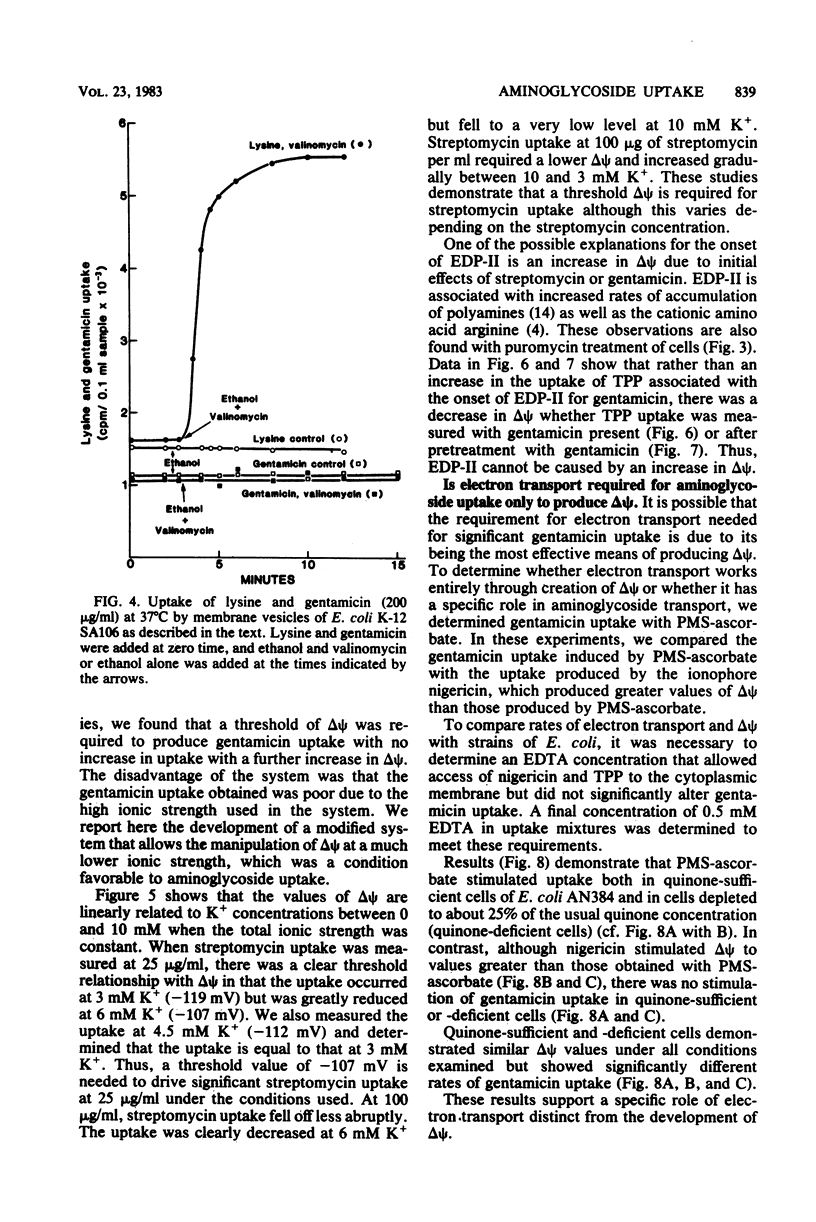
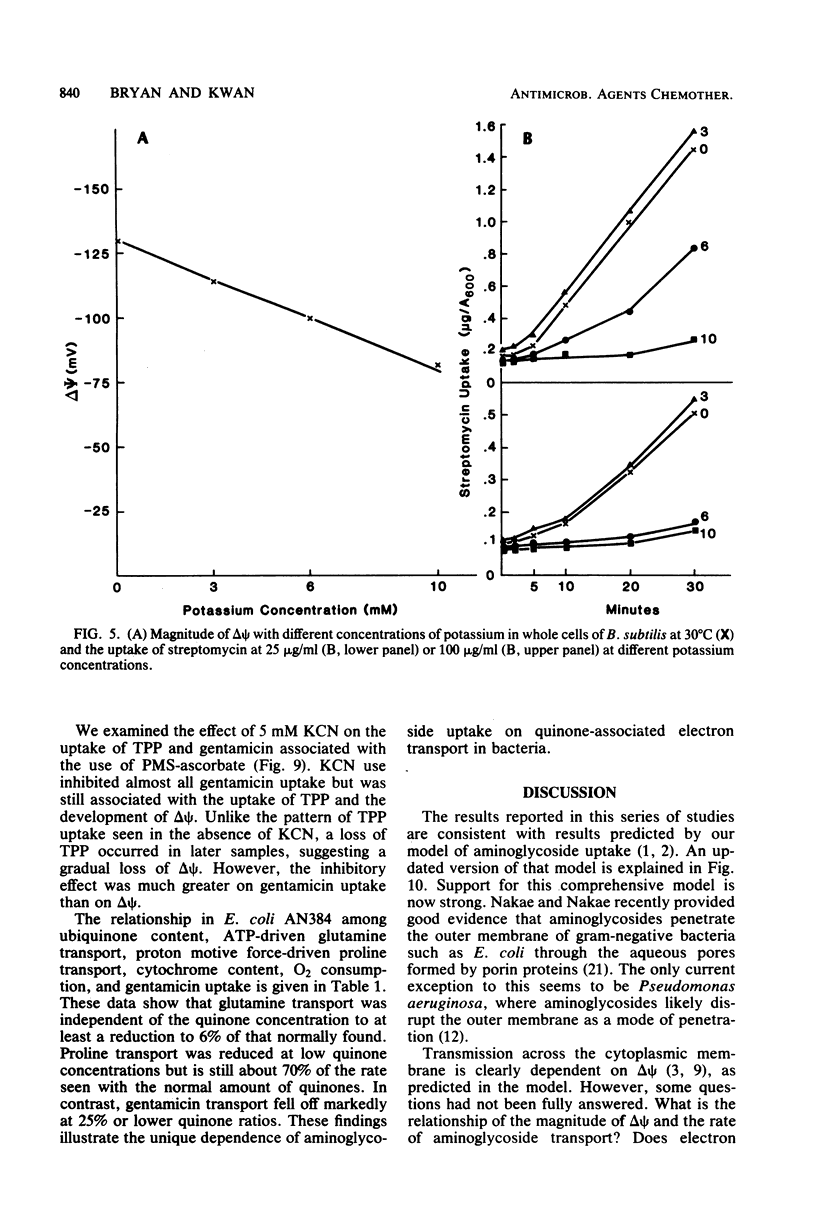

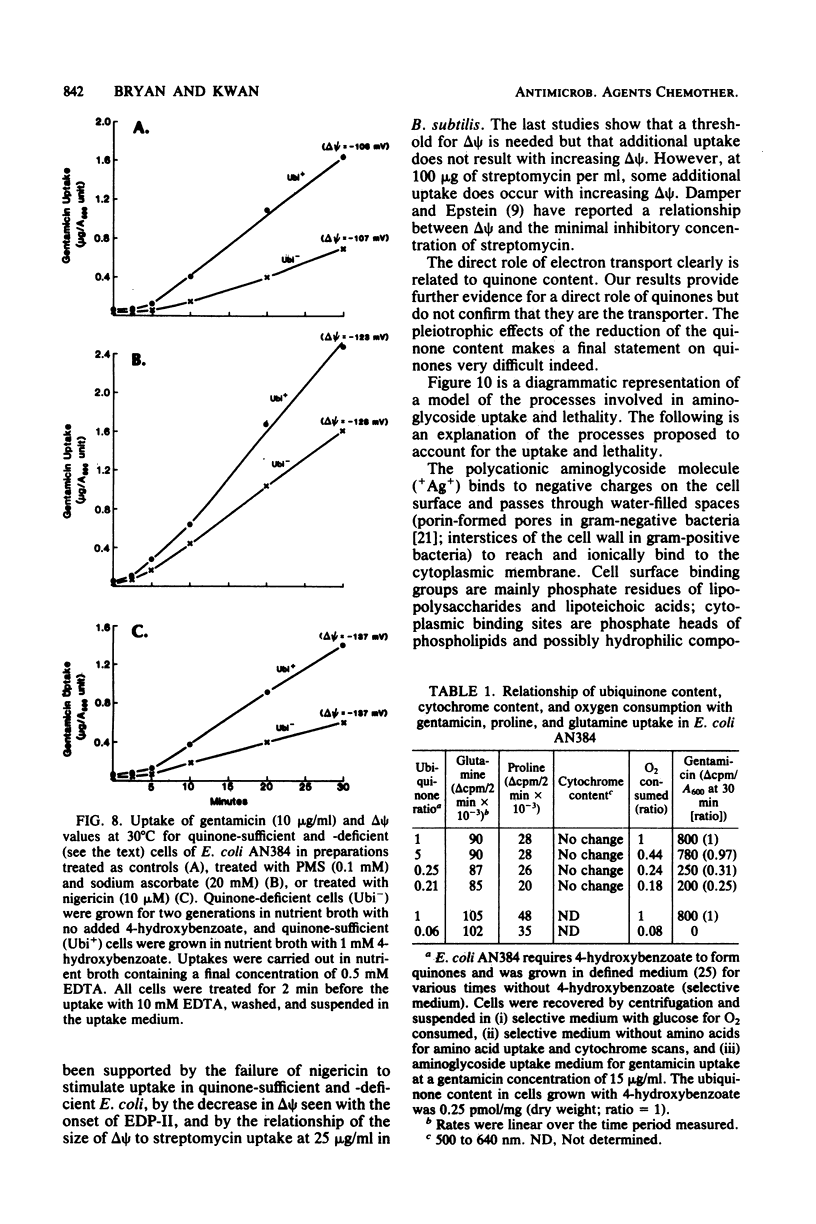


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad M. H., Rechenmacher A., Böck A. Interaction between aminoglycoside uptake and ribosomal resistance mutations. Antimicrob Agents Chemother. 1980 Nov;18(5):798–806. doi: 10.1128/aac.18.5.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan L. E., Kwan S. Mechanisms of aminoglycoside resistance of anaerobic bacteria and facultative bacteria grown anaerobically. J Antimicrob Chemother. 1981 Dec;8 (Suppl 500):1–8. doi: 10.1093/jac/8.suppl_d.1. [DOI] [PubMed] [Google Scholar]
- Bryan L. E., Nicas T., Holloway B. W., Crowther C. Aminoglycoside-resistant mutation of Pseudomonas aeruginosa defective in cytochrome c552 and nitrate reductase. Antimicrob Agents Chemother. 1980 Jan;17(1):71–79. doi: 10.1128/aac.17.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan L. E., Van Den Elzen H. M. Gentamicin accumulation by sensitive strains of Escherichia coli and Pseudomonas aeruginosa. J Antibiot (Tokyo) 1975 Sep;28(9):696–703. doi: 10.7164/antibiotics.28.696. [DOI] [PubMed] [Google Scholar]
- Bryan L. E., Van den Elzen H. M. Streptomycin accumulation in susceptible and resistant strains of Escherichia coli and Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1976 Jun;9(6):928–938. doi: 10.1128/aac.9.6.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell B. D., Kadner R. J. Relation of aerobiosis and ionic strength to the uptake of dihydrostreptomycin in Escherichia coli. Biochim Biophys Acta. 1980 Nov 5;593(1):1–10. doi: 10.1016/0005-2728(80)90002-x. [DOI] [PubMed] [Google Scholar]
- Chopra I., Ball P. Transport of antibiotics into bacteria. Adv Microb Physiol. 1982;23:183–240. doi: 10.1016/s0065-2911(08)60338-0. [DOI] [PubMed] [Google Scholar]
- DUBIN D. T., HANCOCK R., DAVIS B. D. THE SEQUENCE OF SOME EFFECTS OF STREPTOMYCIN IN ESCHERICHIA COLI. Biochim Biophys Acta. 1963 Aug 13;74:476–489. doi: 10.1016/0006-3002(63)91390-8. [DOI] [PubMed] [Google Scholar]
- Damper P. D., Epstein W. Role of the membrane potential in bacterial resistance to aminoglycoside antibiotics. Antimicrob Agents Chemother. 1981 Dec;20(6):803–808. doi: 10.1128/aac.20.6.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickie P., Bryan L. E., Pickard M. A. Effect of enzymatic adenylylation on dihydrostreptomycin accumulation in Escherichia coli carrying an R-factor: model explaining aminoglycoside resistance by inactivating mechanisms. Antimicrob Agents Chemother. 1978 Oct;14(4):569–580. doi: 10.1128/aac.14.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E. Aminoglycoside uptake and mode of action--with special reference to streptomycin and gentamicin. I. Antagonists and mutants. J Antimicrob Chemother. 1981 Oct;8(4):249–276. doi: 10.1093/jac/8.4.249. [DOI] [PubMed] [Google Scholar]
- Hirata H., Altendorf K., Harold F. M. Role of an electrical potential in the coupling of metabolic energy to active transport by membrane vesicles of Escherichia coli. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1804–1808. doi: 10.1073/pnas.70.6.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz C., Braun C. B., Rosano C. L. Role of ribosome recycling in uptake of dihydrostreptomycin by sensitive and resistant Escherichia coli. Biochim Biophys Acta. 1981 Jan 29;652(1):168–176. doi: 10.1016/0005-2787(81)90220-3. [DOI] [PubMed] [Google Scholar]
- Höltje J. V. Streptomycin uptake via an inducible polyamine transport system in Escherichia coli. Eur J Biochem. 1978 May 16;86(2):345–351. doi: 10.1111/j.1432-1033.1978.tb12316.x. [DOI] [PubMed] [Google Scholar]
- Kaback H. R. The role of the phosphoenolpyruvate-phosphotransferase system in the transport of sugars by isolated membrane preparations of Escherichia coli. J Biol Chem. 1968 Jul 10;243(13):3711–3724. [PubMed] [Google Scholar]
- Kashket E. R. Effects of aerobiosis and nitrogen source on the proton motive force in growing Escherichia coli and Klebsiella pneumoniae cells. J Bacteriol. 1981 Apr;146(1):377–384. doi: 10.1128/jb.146.1.377-384.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae R., Nakae T. Diffusion of aminoglycoside antibiotics across the outer membrane of Escherichia coli. Antimicrob Agents Chemother. 1982 Oct;22(4):554–559. doi: 10.1128/aac.22.4.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton N. A., Cox G. B., Gibson F. Function of ubiquinone in Escherichia coli: a mutant strain forming a low level of ubiquinone. J Bacteriol. 1972 Jan;109(1):69–73. doi: 10.1128/jb.109.1.69-73.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlin M. H., Lerner S. A. Localization of an amikacin 3'-phosphotransferase in Escherichia coli. J Bacteriol. 1981 Aug;147(2):320–325. doi: 10.1128/jb.147.2.320-325.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioi J. I., Matsuura S., Imae Y. Quantitative measurements of proton motive force and motility in Bacillus subtilis. J Bacteriol. 1980 Dec;144(3):891–897. doi: 10.1128/jb.144.3.891-897.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace B. J., Young I. G. Role of quinones in electron transport to oxygen and nitrate in Escherichia coli. Studies with a ubiA- menA- double quinone mutant. Biochim Biophys Acta. 1977 Jul 7;461(1):84–100. doi: 10.1016/0005-2728(77)90071-8. [DOI] [PubMed] [Google Scholar]
- Weiss R. L. Protoplast formation in Escherichia coli. J Bacteriol. 1976 Nov;128(2):668–670. doi: 10.1128/jb.128.2.668-670.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


