Abstract
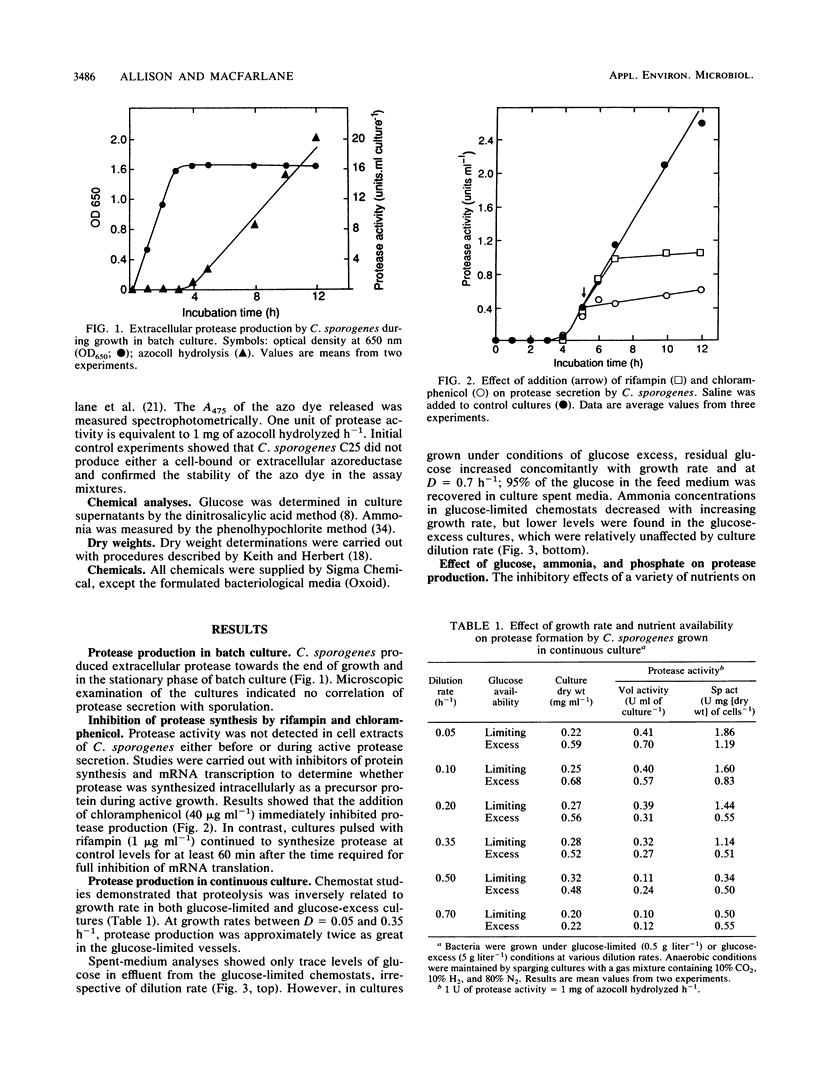
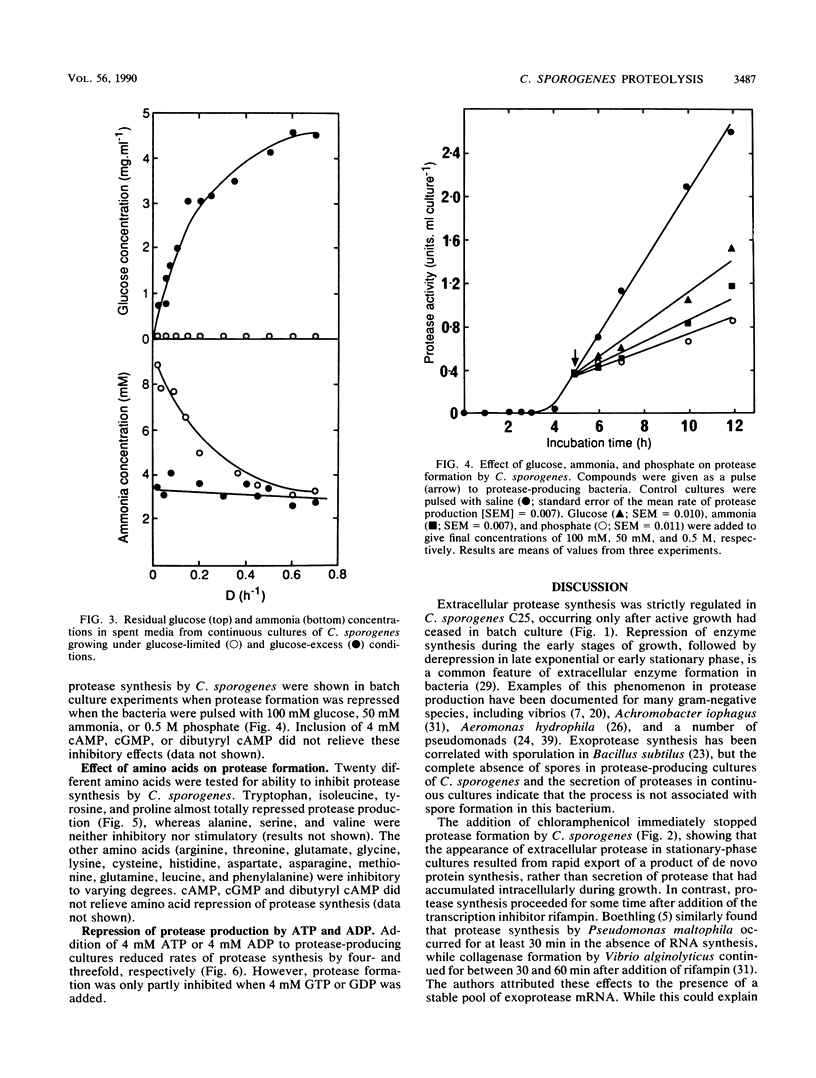
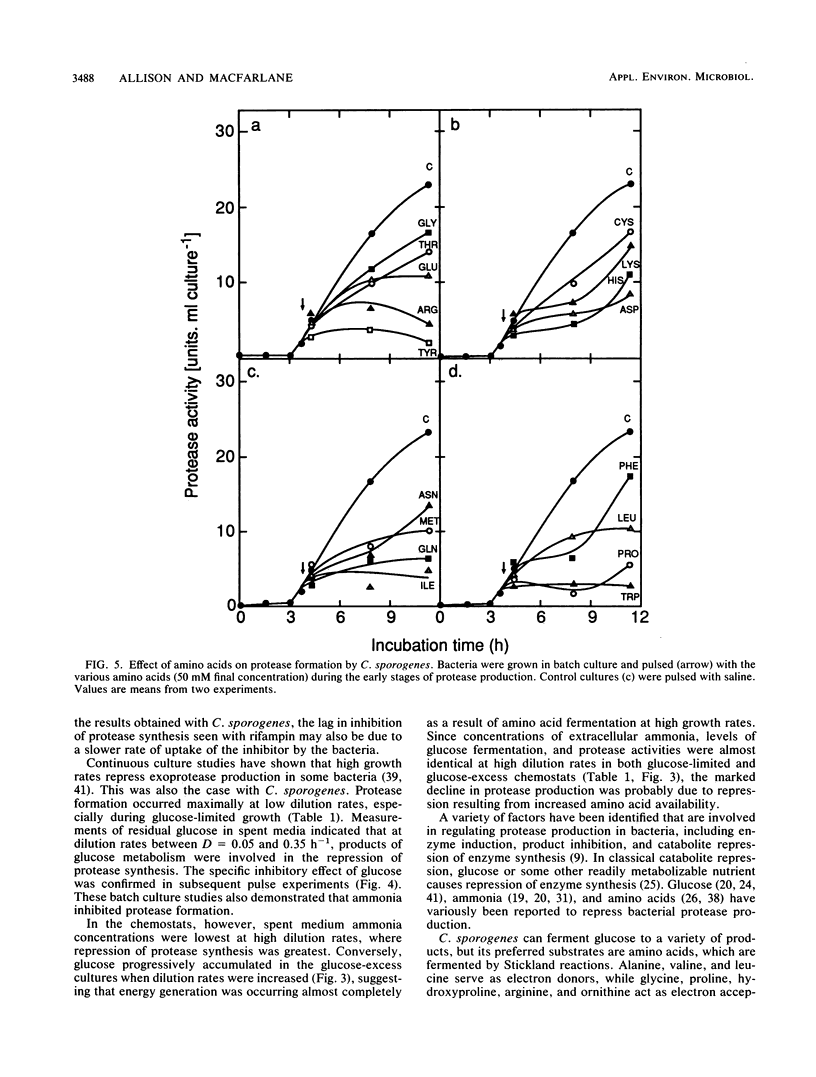
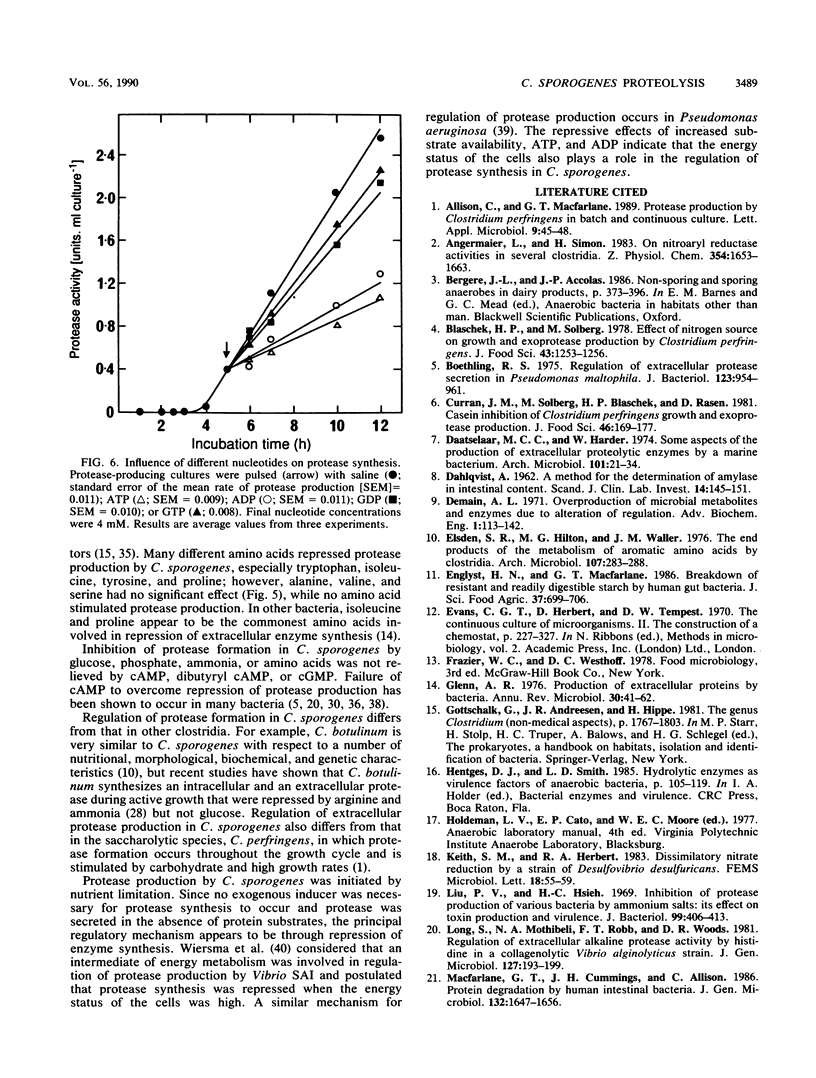
The physiological and nutritional factors that regulate protease synthesis in Clostridium sporogenes C25 were studied in batch and continuous cultures. Formation of extracellular proteases occurred at the end of active growth and during the stationary phase in batch cultures. Protease production was inversely related to growth rate in glucose-excess and glucose-limited chemostats over the range D = 0.05 to 0.70 h-1. In pulse experiments, glucose, ammonia, phosphate, and some amino acids (tryptophan, proline, tyrosine, and isoleucine) strongly repressed protease synthesis. This repression was not relieved by addition of 4 mM cyclic AMP, cyclic GMP, or dibutyryl cyclic AMP. Protease formation was markedly inhibited by 4 mM ATP and ADP, but GTP and GDP had little effect on the process. It is concluded that protease production by C. sporogenes is strongly influenced by the amount of energy available to the cells, with the highest levels of protease synthesis occurring under energy-limiting conditions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angermaier L., Simon H. On nitroaryl reductase activities in several Clostridia. Hoppe Seylers Z Physiol Chem. 1983 Dec;364(12):1653–1663. doi: 10.1515/bchm2.1983.364.2.1653. [DOI] [PubMed] [Google Scholar]
- Bergère J. L., Accolas J. P. Non-sporing and sporing anaerobes in dairy products. Soc Appl Bacteriol Symp Ser. 1986;13:373–396. [PubMed] [Google Scholar]
- Boethling R. S. Regulation of extracellular protease secretion in Pseudomonas maltophilia. J Bacteriol. 1975 Sep;123(3):954–961. doi: 10.1128/jb.123.3.954-961.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAHLQVIST A. A method for the determination of amylase in intestinal content. Scand J Clin Lab Invest. 1962;14:145–151. doi: 10.3109/00365516209079686. [DOI] [PubMed] [Google Scholar]
- Daatselaar M. C., Harder W. Some aspects of the regulation of the production of extracellular proteolytic enzymes by a marine bacterium. Arch Microbiol. 1974;101(1):21–34. doi: 10.1007/BF00455922. [DOI] [PubMed] [Google Scholar]
- Elsden S. R., Hilton M. G., Waller J. M. The end products of the metabolism of aromatic amino acids by Clostridia. Arch Microbiol. 1976 Apr 1;107(3):283–288. doi: 10.1007/BF00425340. [DOI] [PubMed] [Google Scholar]
- Glenn A. R. Production of extracellular proteins by bacteria. Annu Rev Microbiol. 1976;30:41–62. doi: 10.1146/annurev.mi.30.100176.000353. [DOI] [PubMed] [Google Scholar]
- Liu P. V., Hsieh H. C. Inhibition of protease production of various bacteria by ammonium salts: its effect on toxin production and virulence. J Bacteriol. 1969 Aug;99(2):406–413. doi: 10.1128/jb.99.2.406-413.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long S., Mothibeli M. A., Robb F. T., Woods D. R. Regulation of extracellular alkaline protease activity by histidine in a collagenolytic Vibrio alginolyticus strain. J Gen Microbiol. 1981 Nov;127(1):193–199. doi: 10.1099/00221287-127-1-193. [DOI] [PubMed] [Google Scholar]
- MACLENNAN J. D. The histotoxic clostridial infections of man. Bacteriol Rev. 1962 Jun;26:177–276. [PMC free article] [PubMed] [Google Scholar]
- Macfarlane G. T., Cummings J. H., Allison C. Protein degradation by human intestinal bacteria. J Gen Microbiol. 1986 Jun;132(6):1647–1656. doi: 10.1099/00221287-132-6-1647. [DOI] [PubMed] [Google Scholar]
- Mandelstam J., Waites W. M. Sporulation in Bacillus subtilis. The role of exoprotease. Biochem J. 1968 Oct;109(5):793–801. doi: 10.1042/bj1090793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKellar R. C. Factors influencing the production of extracellular proteinase by Pseudomonas fluorescens. J Appl Bacteriol. 1982 Dec;53(3):305–316. doi: 10.1111/j.1365-2672.1982.tb01276.x. [DOI] [PubMed] [Google Scholar]
- Parsons L. B., Sturges W. S. QUANTITATIVE ASPECTS OF THE METABOLISM OF ANAEROBES I. PROTEOLYSIS BY CLOSTRIDIUM PUTREFACIENS COMPARED WITH THAT OF OTHER ANAEROBES. J Bacteriol. 1927 Sep;14(3):181–192. doi: 10.1128/jb.14.3.181-192.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson-Curtis S. I., Johnson E. A. Regulation of neurotoxin and protease formation in Clostridium botulinum Okra B and Hall A by arginine. Appl Environ Microbiol. 1989 Jun;55(6):1544–1548. doi: 10.1128/aem.55.6.1544-1548.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priest F. G. Extracellular enzyme synthesis in the genus Bacillus. Bacteriol Rev. 1977 Sep;41(3):711–753. doi: 10.1128/br.41.3.711-753.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon H., Günther H., Bader J., Neumann S. Chiral products from non-pyridine nucleotide-dependent reductases and methods for NAD(P)H regeneration. Ciba Found Symp. 1985;111:97–111. doi: 10.1002/9780470720929.ch8. [DOI] [PubMed] [Google Scholar]
- Stickland L. H. Studies in the metabolism of the strict anaerobes (genus Clostridium): The chemical reactions by which Cl. sporogenes obtains its energy. Biochem J. 1934;28(5):1746–1759. doi: 10.1042/bj0281746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson M. W., Merrick J. M. Extracellular enzyme secretion by Pseudomonas lemoignei. J Bacteriol. 1974 Jul;119(1):152–161. doi: 10.1128/jb.119.1.152-161.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S., Seifter S. New culture conditions for Clostirdium histolyticum leading to production of collagenase of high specific activity. J Appl Bacteriol. 1972 Dec;35(4):647–657. doi: 10.1111/j.1365-2672.1972.tb03746.x. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Iuchi S. Induction and repression of an extracellular proteinase in Vibrio parahaemolyticus. Biken J. 1971 Jun;14(2):81–96. [PubMed] [Google Scholar]
- Whooley M. A., O'Callaghan J. A., McLoughlin A. J. Effect of substrate on the regulation of exoprotease production by Pseudomonas aeruginosa ATCC 10145. J Gen Microbiol. 1983 Apr;129(4):981–988. doi: 10.1099/00221287-129-4-981. [DOI] [PubMed] [Google Scholar]
- Wiersma M., Hansen T. A., Harder W. Effect of environmental conditions on the production of two extracellular proteolytic enzymes by Vibrio SA1. Antonie Van Leeuwenhoek. 1978;44(2):129–140. doi: 10.1007/BF00643216. [DOI] [PubMed] [Google Scholar]
- Wiersma M., Harder W. A continuous culture study of the regulation of extracellular protease production in Vibrio SA1. Antonie Van Leeuwenhoek. 1978;44(2):141–155. doi: 10.1007/BF00643217. [DOI] [PubMed] [Google Scholar]


