Abstract
Normal production of RBCs requires that the antiapoptotic protein Bcl-xl be induced at end stages of differentiation in response to erythropoietin (Epo) signaling. The critical proapoptotic pathways inhibited by Bcl-xl in erythroblasts are unknown. We used gene targeting in the mouse to evaluate the BH3-only factor Nix, which is transcriptionally up-regulated during Epo-stimulated in vitro erythrocyte differentiation. Nix null mice are viable and fertile. Peripheral blood counts revealed a profound reticulocytosis and thrombocytosis despite normal serum Epo levels and blood oxygen tension. Nix null mice exhibited massive splenomegaly, with splenic and bone marrow erythroblastosis and reduced apoptosis in vivo during erythrocyte maturation. Hematopoietic progenitor populations were unaffected. Cultured Nix null erythroid cells were hypersensitive to Epo and resistant to apoptosis stimulated by cytokine deprivation and calcium ionophore. Transcriptional profiling of Nix null spleens revealed increased expression of cell cycle and erythroid genes, including Bcl-xl, and diminished expression of cell death and B cell-related genes. Thus, cell-autonomous Nix-mediated apoptosis in opposition to the Epo-induced erythroblast survival pathway appears indispensable for regulation of erythrocyte production and maintenance of hematological homeostasis. These results suggest that physiological codependence and coordinated regulation of pro- and antiapoptotic Bcl2 family members may represent a general regulatory paradigm in hematopoiesis.
Keywords: apoptosis, Bcl2 proteins, erythropoietin, polycythemia vera
The mammalian hematopoietic system replaces blood elements as they age and in response to physiological demands, and homeostasis is maintained by balancing stem cell proliferation with commitment to and differentiation of hematopoietic lineages. Hypoxia is the major physiological stimulus for RBC production (erythropoiesis), stimulating production of erythropoietin (Epo) by the adult kidney and fetal liver. Epo controls erythrocyte production (1) by preventing apoptosis (2, 3) through activation of Janus kinase 2 (JAK2) and Stat5 (4), which induce expression of the antiapoptotic Bcl2 family member Bcl-xl (5). Epo/Bcl-xl-dependent survival is both necessary and sufficient for terminal erythroid differentiation (6). Consequently, in mouse models, absence of Epo or its receptor (1, 7), the Epo effector, Stat5 (5), or the Epo/Stat5 target, Bcl-xl (8, 9), results in apoptosis of erythrocyte progenitors and anemia.
Increased Epo levels (10) or receptor mutations that cause hypersensitivity to Epo (11) cause human erythropoietic disorders. Polycythemia vera, the prototypical erythopoietic disease, is associated with various somatic mutations of JAK2 (12–14). Other hallmarks of polycythemia vera include increased Bcl-xl expression and Epo-independent erythroblast growth (15). Because the antiapoptotic effects of Bcl-xl occur by sequestration and inhibition of proapoptotic BH3-only proteins that activate Bax and Bak (16–18), one or more proapoptotic BH3-only proteins may be involved in erythropoiesis.
Peripheral blood cells undergoing Epo-induced erythroid differentiation exhibit concurrent transcriptional up-regulation of antiapoptotic Bcl-xl and the proapoptotic BH3-only-like protein Nix (19). Because Bcl-xl can bind to and inhibit Nix (20), we hypothesized that Nix-mediated cell death signaling could regulate erythropoiesis in opposition to survival signaling by Epo and Bcl-xl. Accordingly, we used gene ablation of Nix in mice and found that Nix is a specific and essential negative regulator of erythropoiesis through modulated apoptosis.
Results
Nix Causes Mitochondrial Outer Membrane Permeabilization, But Not Opening of the Permeability Transition Pore.
Nix causes mitochondrial pathway apoptosis in transfected cells (21, 22) and is inhibited by Bcl-xl (20). A proapoptotic factor acting in opposition to Bcl-xl in erythropoiesis must be able to initiate mitochondrial pathway apoptosis. We compared the ability of Nix to promote mitochondrial outer membrane permeabilization and opening of the mitochondrial permeability transition pore to that of Bax (18) and to an inactive C-terminal truncation mutant of Nix, sNix, that is not targeted to mitochondria (22). Cytochrome c, which activates caspases in combination with cytosolic factors (23), was released from mitochondria by recombinant GST-Nix, but not mitochondrial-defective GST-sNix (Fig. 1 A and B). GST-Nix did not cause mitochondrial transition pore opening, assessed as mitochondrial swelling (24), nor did it sensitize mitochondria to swelling induced by Ca2+ (Fig. 1C). Previously, we have shown that recombinant Nix expression in cultured HEK293 cells induces cytochrome c release, activates caspase 3, and produces apoptosis (TUNEL labeling) (22). Thus, Nix is sufficient to initiate apoptosis via mitochondrial cytochrome c release, likely in combination with intrinsic mitochondrial proteins (25).
Fig. 1.
Nix effects on isolated mitochondria and general phenotype of Nix gene ablation in mice. (A) Isolated WT mouse liver mitochondria were incubated with increasing concentrations of GST-Nix, GST-sNix, and GST-Bax. Resultant mitochondrial pellet and supernatant underwent Western blotting for cytochrome c and cytochrome oxidase IV (COX-IV) (n = 3). (B) Time-course studies (0–180 min) as in A. (C) Swelling of isolated WT mitochondria induced by GST-Nix, 250 μM Ca2+, and GST-Nix + 250 μM Ca2+ (means of n = 2). (D) Schematic of Nix deletion strategy. Exons 1–6b (filled rectangles) and restriction sites are depicted (see SI Methods). (E) Southern blot (Left) and PCR (Right) screening of Nix-targeted mice. (F) Multiple-tissue Northern blot hybridized to Nix probes. (G) Hypomorphism. (H) Splenomegaly of WT (Upper) and Nix−/− (Lower) mice.
Splenomegaly and Erythrocyte Abnormalities in Nix-Deficient Mice.
Published data have described low basal levels of Nix gene expression in most tissues, with transcriptional Nix up-regulation under conditions of cell stress, growth, or differentiation (19, 22, 26, 27). Accordingly, we carried out germ-line deletion of Nix in mice (Nix−/− mice) by flanking exons 4 through 6a of the Nix gene with loxP sites and then breeding to mice expressing Cre recombinase driven by zygotically expressed adenovirus EIIa (28) (Fig. 1D). Genotyping was by Southern analysis and PCR (Fig. 1E). Interbreeding of heterozygous (+/−) mice produced healthy and fertile offspring, with normal Mendelian inheritance of the mutant gene (Nix: 15/80+/+, 45/80+/−, 20/80−/−). Nix protein was not detectable in any normal mouse tissue by immunoblot analysis (data not shown), consistent with its rapid proteasomal degradation (19). However, Nix mRNA, which is visualized as ≈1.5- and ≈4.0-kb species due to the presence of two polyadenlyation sites (22, 27), was absent from organs of knockout mice (Fig. 1F).
Nix−/− mice survive to at least 18 months of age, but are externally distinguishable from WT mice by modest growth retardation (Fig. 1G), with a 16 ± 6% reduction in body mass (P = 0.04) and a 9 ± 3% reduction in nose-to-anus length (n = 7 pairs, P = 0.03) at 12 weeks. No organ abnormalities were observed [supporting information (SI) Table 2], except striking enlargement of Nix−/− spleens, which averaged ≈80% heavier in 8-week-old mice (8.4 ± 2 mg/g body weight Nix−/−, compared with 4.8 ± 0.9 mg/g body weight in WT; n = 10 each, P < 0.001) (Fig. 1H) and further increasing to ≈250% heavier at 40 weeks of age (12.1 ± 1.1 mg/g Nix−/− vs. 4.9 ± 0.6 mg/g in WT; n = 6 each, P = 0.001; P = 0.015 vs. 8-week-old Nix−/− mice).
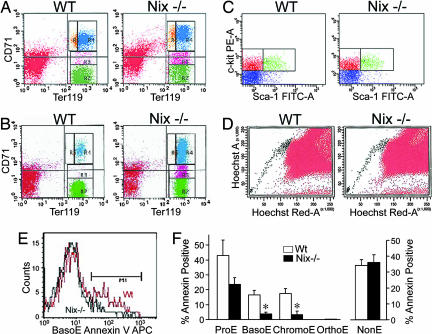
The cellular makeup of Nix−/− spleens was determined by comparative histology and immunophenotyping. There were no differences in splenic or thymic lymphocyte subpopulations (SI Fig. 7). Germinal centers were histologically normal, but the red pulp was increased (Fig. 2 A and B), and staining for glycophorin-A-associated Ter119, a marker of erythroid cells (29), was increased (Fig. 2C). Flow-cytometric analysis showed that Nix−/− mice had, proportionally, 27% more Ter119+ splenocytes (66 ± 5% of total) than WT (52 ± 3% of total; n = 6 paired studies, P = 0.035) (Fig. 2D). When corrected for increased spleen size in Nix−/− mice, this reflects a 2.3- to 3.2-fold increase in spleen erythroblast mass. Blood levels of Epo were not elevated in Nix−/− mice (325 ± 33 pg/ml vs. 317 ± 45 in WT; n = 6 pairs, P = 0.885; normal range <683 pg/ml), and blood oxygen saturation determinations obtained on anesthetized, room air-ventilated mice were not decreased (94 ± 2% in Nix−/− vs. 91 ± 1% in WT; n = 4 pairs), indicating that erythroblastosis was due to an intrinsic defect in control of erythroblast numbers. Peripheral blood counts of Nix−/− mice revealed reticulocytosis and thrombocytosis (Table 1) with poikilocytosis, polychromasia, and anisocytosis of erythrocytes (Fig. 2E), and increased erythrocyte volume (Table 1). Because reticulocytes are larger than mature erythrocytes, and reticulocytosis could therefore skew determinations of mean corpuscular volume (MCV), this parameter was independently determined for erythrocytes and reticulocytes. In WT, mature erythrocyte MCV was 45.9 ± 0.5 fl and for reticulocytes was 51.1 ± 0.7 fl; for Nix−/− mice, mature erythrocyte MCV was 49.4 ± 0.5 fl and for reticulocytes was 55.9 ± 0.6 fl (P = 0.002 for both measurements).
Fig. 2.
Splenic erythroblastosis and erythrocyte abnormalities in Nix−/− mice. (A and B) H&E-stained splenic sections. (Magnification: A, ×4; B, ×20.) (C) Ter119-stained (brown) splenic sections. Blue is counterstained lymphoid tissue. (D) Representative flow-cytometric quantification of Ter119+ splenocytes. (E) Wright-Giemsa-stained peripheral blood smears (1, polychromatic cells; 2, immature erythrocytes with redundant membrane; 3, discocytes).
Table 1.
Hematopoietic cell counts of Nix−/− mice
| WT | Nix−/− | Pvalue/stats | |
|---|---|---|---|
| Blood | |||
| WBC, 1,000/μl | 7.9 ± 0.7 | 9.6 ± 1.6 | 0.294 |
| Neutrophils, % | 13 ± 1 | 15 ± 4 | 0.508 |
| Lymphocytes, % | 84 ± 6 | 80 ± 3 | 0.242 |
| RBC (1,000,000/μl) | 9.6 ± 0.7 | 7.7 ± 0.2* | 0.001 |
| Hb, g/dl | 14.4 ± 1.1 | 13.3 ± 0.2* | 0.005 |
| Hct, % | 48.5 ± 3.6 | 45.6 ± 0.9 | 0.055 |
| Reticulocytes, % | 5 ± 0.4 | 14 ± 1.1* | <0.001 |
| MCV, fl | 50 ± 4 | 59 ± 0.1* | <0.001 |
| Platelets, 1,000/μl | 835 ± 85 | 1,291 ± 30* | <0.001 |
| Bone marrow | |||
| Total Ter119 + cells, % | 47 ± 2 | 44 ± 3 | 0.580 |
| Proerythroblast, % | 2 ± 1 | 2 ± 1 | 0.772 |
| Baso. erythroblast, % | 16 ± 3 | 25 ± 2* | 0.036 |
| Chro. erythroblast, % | 2 ± 1 | 4 ± 1* | 0.025 |
| Orth. erythroblast, % | 27 ± 2 | 13 ± 2* | 0.001 |
| Spleen | |||
| Total Ter119 + cells, % | 54 ± 7 (9) | 66 ± 12 (6)* | 0.025 |
| Proerythroblast, % | 0.1 ± 0.1 | 0.5 ± 0.1* | 0.007 |
| Baso. erythroblast, % | 2 ± 1 | 23 ± 3* | 0.004 |
| Chro. erythroblast, % | 1 ± 1 | 10 ± 1* | 0.001 |
| Orth. erythroblast, % | 53 ± 3 | 28 ± 3* | 0.001 |
All data are mean ± SEM (n). Statistical test results are reported as P value by t test. The numbers of WT and Nix−/−, respectively, are as follows: blood, 12 and 10; bone marrow, 5 and 5; and spleen, 7 and 4.
*P < 0.05 vs. WT.
Nix-Deficient Spleens and Bone Marrow Show Erythroblast Hyperplasia Associated with Reduced Apoptosis.
Erythroblast maturation follows from proerythroblast (CD71 high, Ter119 mid) to basophilic erythroblast (CD71 high, Ter119 high) to late basophilic and chromatophilic erythroblasts (CD71 mid, Ter119 high) to orthochromatic erythroblasts (CD71 low, Ter119 high) (30). Nix−/− bone marrow had significantly greater numbers of chromatophilic and basophilic erythroblasts, with proportionally fewer orthochromatic erythroblasts than WT (Table 1 and Fig. 3A). Likewise, Nix−/− spleens showed increased erythroblasts throughout the early and mid-erythrocyte maturation sequence and a proportional reduction in orthochromatic erythroblasts (Table 1 and Fig. 3B).
Fig. 3.
Erythroblast hyperplasia and diminished apoptosis in Nix−/− bone marrow and spleens. (A and B) Ter119 and CD71 expression in freshly isolated bone marrow (A) or splenic (B) cells. Cells: yellow, proerythroblasts (ProE); blue, basophilic erythroblasts (BasoE); pink, chromatophilic erythroblasts (ChromoE); green, orthochromatic erythroblasts (OrthoE). (C and D) Representative flow-cytometric analysis of Lin-, Sca-1/c-kit+ fraction (C), and Hoechst 33342-excluding “side population” (D) bone marrow cells. (E and F) Analysis of in vivo apoptosis in splenocytes. NonE, nonerythroblasts; ∗, P < 0.05.
Accumulation of early and mid-erythroblasts in Nix−/− mice could reflect increased numbers of hematopoietic progenitor cells. To address this, hematopoietic progenitor cells were quantified as lineage-negative, Sca-1- and c-kit-positive fraction in adult mouse bone marrow (31) (Fig. 3C), or as the “side population” of bone marrow cells that exclude Hoechst 33342 due to the absence of the multidrug resistance protein 1 efflux pump (32) (Fig. 3D). By either measure, the relative proportion of hematopoietic stem cells/progenitor cells to total nucleated bone marrow cells was not significantly altered in Nix−/− mice (side population, 0.092 ± 0.015% Nix−/− vs. 0.065 ± 0.013% WT; n = 6 pairs, P = 0.203; Lin-, Sca-1/c-kit+, 2.50 ± 0.74% Nix−/− vs. 2.98 ± 0.77% WT; n = 5 pairs, P = 0.67). These results indicate that erythroblastosis in Nix−/− mice is not due to increased hematopoietic progenitors.
To determine whether committed erythroblasts accumulated due to reduced cell death, we examined externalization of phosphatidylserine by Annexin V staining on the surface of Ter119+ bone marrow cells and splenocytes. Annexin V labeling was significantly decreased in basophilic (Fig. 3 E and F) and chromatophilic erythroblasts from Nix−/− spleens (Fig. 3F) and in chromatophilic erythroblasts from Nix−/− bone marrow (3 ± 1% Nix−/− vs. 16 ± 4% WT; n = 4, P = 0.008), compared with WT. Propidium iodide staining of dead cells gave similar results (SI Fig. 8). Nonerythroid cells showed no difference in Annexin V labeling (Fig. 3F Right). Together these results indicate that baso- and chromatophilic erythroblasts accumulate in Nix−/− mice due to reduced cell death, suggesting that Nix regulates apoptosis during erythroid differentiation.
Nix−/− Splenocytes Are Hyperresponsive to Epo.
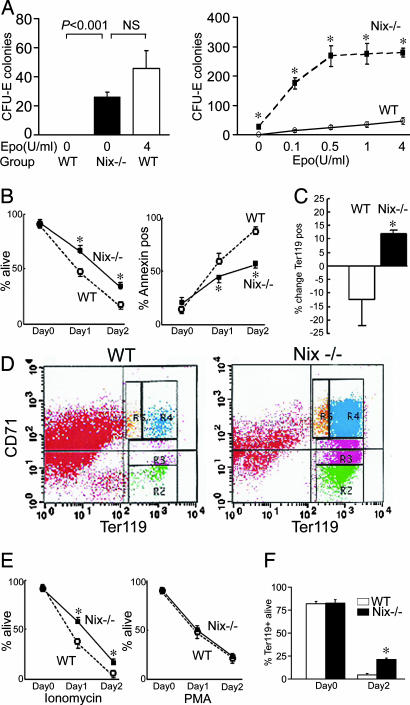
We examined the sensitivity of Nix−/− erythroblasts to Epo by using in vitro colony assays for colony-forming unit-erythroid (CFU-E). In the absence of exogenous Epo, there was no CFU-E colony formation in cultured WT splenocytes. In contrast, Nix−/− splenocytes produced CFU-E colonies in the absence of exogenous Epo at numbers approximating that for maximally Epo-stimulated WT splenocytes (Fig. 4A), revealing Epo-independent erythroid development. With increasing doses of Epo, CFU-E colony formation averaged an order of magnitude greater than WT at the same Epo doses (Fig. 4A).
Fig. 4.
Epo-hyperresponsiveness and apoptosis resistance of Nix−/− splenocytes. (A) CFU-E colony formation with and without increasing doses of Epo (n = 6–7 paired experiments; ∗, P ≤ 0.001 compared with WT). (B) Survival (Left, n = 5) and apoptosis (Right, n = 4) of splenocytes in monoculture. (C and D) Proportional change in Ter119+ splenocytes (C) and Ter119 and CD71 expression (D) after 48 h of suspension monoculture as in B (n = 4 paired experiments). (E) Splenocyte survival after apoptotic provocation with ionomycin 1 μg/ml (Left) or PMA 2 ng/ml (Right; n = 5 paired experiments; ∗, P < 0.05). (F) Survival of Ter119+ splenocytes in vitro (n = 4 paired experiments; ∗, P < 0.05).
Nix−/− Erythrocyte Precursors Are Resistant to Multiple Apoptotic Stimuli.
The previous data support the hypothesis that Nix regulates erythropoiesis in a cell-autonomous manner in opposition to cell-survival signaling by Epo/Bcl-xl. To determine whether Nix also regulates erythroblast apoptosis signaling in response to pathological events, we compared the in vitro sensitivity of Nix−/− and WT erythroblasts to different apoptotic stimuli. Splenocytes maintained in conventional monoculture undergo apoptosis due to cytokine deprivation or “death by neglect” (17). Compared with WT, Nix−/− splenocytes in suspension culture showed enhanced survival and diminished rates of apoptosis (Fig. 4B). Immunophenotyping of surviving WT and Nix−/− splenocytes showed enrichment of Nix−/− cells in the erythrocytic lineage (Fig. 4 C and D). Nix appears to have a role in apoptosis stimulated by some, but not all, factors, because Nix−/− splenocytes were also resistant to death after treatment with ionomycin, but not phorbol ester (Fig. 4E).
Enrichment of Ter119+ cells in mixed splenocytes undergoing cytokine deprivation suggests that it is the Ter119+ erythroid population that is resistant to apoptosis. We tested this notion by presorting WT and Nix−/− Ter119+ splenocytes and repeating the death-by-neglect studies in this erythroid population. Enhanced survival of Nix−/− cells was again observed (Fig. 4F).
Molecular Signatures of Hematopoiesis, Cell Cycling, and Death in Nix−/− Spleens.
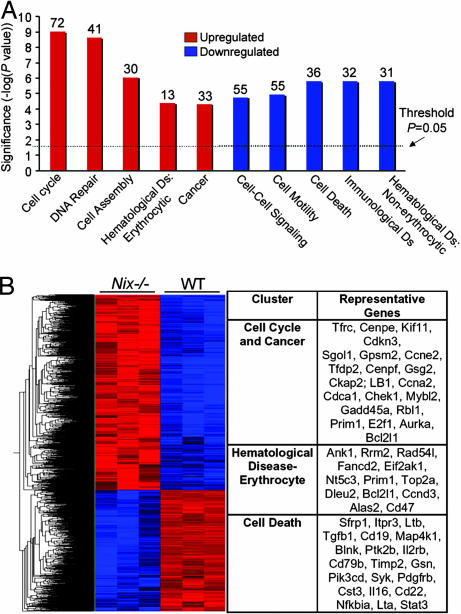
We used a nonbiased approach to assess the consequences of Nix ablation on apoptosis and erythropoiesis by using comparative transcriptional profiling. Of 45,101 genes analyzed, 514 were significantly up-regulated and 386 were down-regulated in Nix−/− splenocytes, compared with WT. Functional clustering revealed increased expression of cell cycle and erythroid genes, with decreased levels of cell death and B cell genes (Fig. 5 A and B). Notable among up-regulated genes is Bcl-xl (increased 2.7-fold) and the erythrocytic markers transferrin receptor (CD71; increased 3.2-fold) and glycophorin A (increased 3.7-fold). These results independently show that Nix ablation is associated with transcriptional markers of splenic erythrocytosis and diminished apoptosis. A complete annotated list of Nix−/−-regulated spleen transcripts within the 10 functional clusters is provided in SI Tables 3 and 4, and the complete data set is available at www.ncbi.nlm.nih.gov/geo (accession no. GSE7020).
Fig. 5.
Altered patterns of gene expression in Nix−/− spleens. (A) Enrichment (red) and disenrichment (blue) of selected functional gene groups in Nix−/− spleens. (B) Dendrogram and heat map depiction (Left) and abbreviated list of regulated genes (Right). Color intensity (red:highest to blue:lowest) displays relative expression.
Discussion
Previous gene-targeting studies of proapoptotic Bcl2 family proteins Bim, Bad, Bax, and Bak, either alone or in combination, found myelo- or lymphoproliferative phenotypes (17, 33–35), showing that a programmed apoptosis checkpoint normally restrains leukocyte formation. Although experimental models and human data relating abnormal activity of the Epo–JAK2–Bcl-xl pathway to clinical erythroproliferative disorders have implied that a similar cell-survival/apoptosis homeostatic mechanism exists for erythropoiesis (6), the critical factor(s) constituting the proapoptotic arm have not been previously identified. The current studies indicate an important role for Nix in this process.
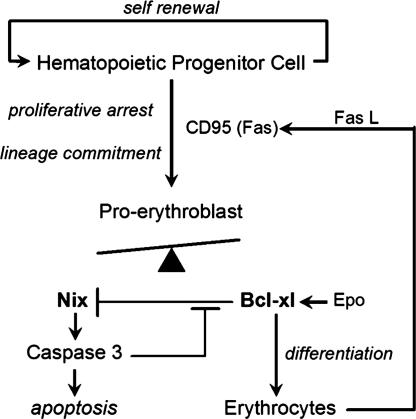
Nix (Nip-like protein X, originally Bnip3L) (21) is a transcriptionally regulated proapoptotic BH3-only-like protein whose expression in cultured human CD34+ blood cells increases along with Bcl-xl during erythroid differentiation (19). Here we show that Nix fulfills the criteria for an apical proapoptotic regulator of normal erythroblast apoptosis. Nix, likely interacting with intrinsic mitochondrial proteins, caused mitochondrial cytochrome c release, which can initiate mitochondrial pathway apoptosis. In mice, Nix ablation diminished apoptosis and caused accumulation of committed erythroblasts, but did not affect hematopoietic progenitor cells. Nix ablation increased expression of erythroid genes while decreasing cell death genes. Thus, Nix appears to function as a critical initiator of an intrinsic apoptosis pathway that is specific, at least among hematopoietic cell lineages, for cells committed to erythrocyte development (Fig. 6).
Fig. 6.
Schematic depiction of Nix involvement in erythroid maturation pathway. Fas L, Fas ligand.
The fact that Nix ablation had no impact on hematopoietic progenitor cell numbers is consistent with the observation that the undifferentiated self-renewing cells in this population do not normally undergo apoptosis (36, 37). The transition of progenitor cells toward a differentiated, nonproliferative state is stimulated in part by an interaction with Fas ligand expressed on the surface of mature erythroblasts (38), establishing the first level of apoptosis-maturational cross-talk during erythropoiesis. Normally, Epo and the primary erythroid transcription factor, GATA-1, cooperate to induce antiapoptotic Bcl-xl gene expression in erythroblasts (39), initiating an essential prosurvival signal leading to RBC formation (Fig. 6). Stimulation of Fas/CD95 death receptors activates caspases that cleave GATA-1, thus exerting a negative effect on erythropoiesis (40). Opposing erythroblast survival and maturation is an intrinsic apoptosis pathway that we suggest is regulated through Nix, which is induced in maturing erythroblasts (19), permeabilizes mitochondrial outer membranes, and releases cytochrome c to activate caspases. In addition to being a terminal effector of apoptosis, caspase 3 may also cleave and inactivate Bcl-xl (41), thus providing positive feedback for Nix-mediated erythroblast apoptosis (Fig. 6). Together these data suggest that the default cell-fate pathway after erythroid commitment is apoptosis, and that generation of erythrocytes requires the active intervention of Epo/Bcl-xl signaling to rescue erythroblasts from Nix-mediated death.
The Nix−/− phenotype is intriguingly similar to polycythemia vera (11), a relatively rare disorder in which the majority of affected individuals carry a somatic-activating mutation of JAK2 that constitutively activates Epo-pathway signaling (12) that may not be sufficient to cause the full-fledged syndrome (42). Hallmark features of polycythemia vera include reticulocytosis and thrombocytosis in the peripheral blood, erythroblastosis of the bone marrow, low circulating Epo levels, and pronounced splenomegaly, which are all seen with Nix ablation. Likewise, both polycythemia vera and Nix ablation are characterized by Epo-independent stimulation of erythroid colony formation (11). Most human subjects with polycythemia vera also have increased RBC numbers or mass not seen with Nix ablation, perhaps due to erythrocyte sequestration in massively enlarged spleens (43).
The specific regulatory function of Nix in erythropoiesis distinguishes it from other BH3-only factors and the multidomain proapoptotic Bcl-2 family members, which, when their genes were ablated individually or in combination, resulted in increased lymphoid cells in the spleen or thymus without affecting the erythroid lineage (17, 33–35, 44, 45). We propose that the apparently unique physiological role of Nix in erythropoiesis stems in part from its tight regulation during the orderly sequence of erythroid maturation. Our results provide specific data to support the general paradigm that coordinate regulation of apoptosis and cell-survival pathways is necessary for homeostasis during normal hematopoiesis and have identified Nix as the critical proapoptotic mediator within the erythroid lineage.
Experimental Procedures
Generation of Nix−/− Mice.
Exons 4 to 6a, encoding the putative BH3 domain and the essential carboxyl-terminal transmembrane domain (22) of Nix, were targeted by flanking them with loxP sites, in combination with a frt-flanked neomycin phosphotransferase module to positively select putative homologous recombinant embryonic stem cells. Correctly targeted recombinants were identified by EcoRI restriction digest and Southern blot, with a 3′ probe external to the targeting vector. Following implantation of embryonic stem cells into blastocysts, generation of chimeric mice, and breeding to the F1 generation, heterozygous Nix-targeted mice were bred with Flp transgenic mice (46) to delete the neomycin-selection cassette. Progeny were crossed to remove the Flp transgene, resulting in mice bearing only floxed Nix alleles (heterozygous Nixf/+; homozygous Nixf/f). EIIa-Cre transgenic mice (28) were bred on to the Nixf/+ background and crossed with Nixf/f mice to generate Nix null mice (Nix−/−). Nix−/− mice were maintained on a mixed 129/C57BL/6 genetic background and were housed and studied according to procedures approved by the University of Cincinnati Institutional Animal Care and Use Committee.
Other detailed methods are provided in SI Methods.
Statistics.
Results are expressed as mean + SEM. Statistical differences were assessed with the unpaired t test for two experimental groups and paired t tests for in vitro cell death assays. A nonparametric test was applied when the data were not normally distributed. Significance was determined by a two-tailed P < 0.05 for t test.
Supplementary Material
Acknowledgments
This work was supported by National Heart, Lung, and Blood Institute Grants HL59888, HL58010, HL77101, and HL69779 (to G.W.D.); and by the U.S. Department of Veterans Affairs (A.D.).
Abbreviations
- Epo
erythropoietin
- MCV
mean corpuscular volume.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GEO7020).
This article contains supporting information online at www.pnas.org/cgi/content/full/0610666104/DC1.
References
- 1.Wu H, Liu X, Jaenisch R, Lodish HF. Cell. 1995;83:59–67. doi: 10.1016/0092-8674(95)90234-1. [DOI] [PubMed] [Google Scholar]
- 2.Koury MJ, Bondurant MC. Science. 1990;248:378–381. doi: 10.1126/science.2326648. [DOI] [PubMed] [Google Scholar]
- 3.Kelley LL, Koury MJ, Bondurant MC, Koury ST, Sawyer ST, Wickrema A. Blood. 1993;82:2340–2352. [PubMed] [Google Scholar]
- 4.Constantinescu SN, Ghaffari S, Lodish HF. Trends Endocrinol Metab. 1999;10:18–23. doi: 10.1016/s1043-2760(98)00101-5. [DOI] [PubMed] [Google Scholar]
- 5.Socolovsky M, Fallon AE, Wang S, Brugnara C, Lodish HF. Cell. 1999;98:181–191. doi: 10.1016/s0092-8674(00)81013-2. [DOI] [PubMed] [Google Scholar]
- 6.Dolznig H, Habermann B, Stangl K, Deiner EM, Moriggl R, Beug H, Mullner EW. Curr Biol. 2002;12:1076–1085. doi: 10.1016/s0960-9822(02)00930-2. [DOI] [PubMed] [Google Scholar]
- 7.Kieran MW, Perkins AC, Orkin SH, Zon LI. Proc Natl Acad Sci USA. 1996;93:9126–9131. doi: 10.1073/pnas.93.17.9126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Motoyama N, Wang F, Roth KA, Sawa H, Nakayama K, Nakayama K, Negishi I, Senju S, Zhang Q, Fujii S, et al. Science. 1995;267:1506–1510. doi: 10.1126/science.7878471. [DOI] [PubMed] [Google Scholar]
- 9.Wagner KU, Claudio E, Rucker EB, III, Riedlinger G, Broussard C, Schwartzberg PL, Siebenlist U, Hennighausen L. Development (Cambridge, UK) 2000;127:4949–4958. doi: 10.1242/dev.127.22.4949. [DOI] [PubMed] [Google Scholar]
- 10.Ang SO, Chen H, Hirota K, Gordeuk VR, Jelinek J, Guan Y, Liu E, Sergueeva AI, Miasnikova GY, Mole D, et al. Nat Genet. 2002;32:614–621. doi: 10.1038/ng1019. [DOI] [PubMed] [Google Scholar]
- 11.Schafer AI. Blood. 2006;107:4214–4222. doi: 10.1182/blood-2005-08-3526. [DOI] [PubMed] [Google Scholar]
- 12.James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, Garcon L, Raslova H, Berger R, Bennaceur-Griscelli A, et al. Nature. 2005;434:1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 13.Scott LM, Tong W, Levine RL, Scott MA, Beer PA, Stratton MR, Futreal PA, Erber WN, McMullin MF, Harrison CN, et al. N Engl J Med. 2007;356:459–468. doi: 10.1056/NEJMoa065202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, Tichelli A, Cazzola M, Skoda RC. N Engl J Med. 2005;352:1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 15.Silva M, Richard C, Benito A, Sanz C, Olalla I, Fernandez-Luna JL. N Engl J Med. 1998;338:564–571. doi: 10.1056/NEJM199802263380902. [DOI] [PubMed] [Google Scholar]
- 16.Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, Korsmeyer SJ. Mol Cell. 2001;8:705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- 17.Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA, Ulrich E, Waymire KG, Mahar P, Frauwirth K, et al. Mol Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aerbajinai W, Giattina M, Lee YT, Raffeld M, Miller JL. Blood. 2003;102:712–717. doi: 10.1182/blood-2002-11-3324. [DOI] [PubMed] [Google Scholar]
- 20.Imazu T, Shimizu S, Tagami S, Matsushima M, Nakamura Y, Miki T, Okuyama A, Tsujimoto Y. Oncogene. 1999;18:4523–4529. doi: 10.1038/sj.onc.1202722. [DOI] [PubMed] [Google Scholar]
- 21.Chen G, Cizeau J, Vande VC, Park JH, Bozek G, Bolton J, Shi L, Dubik D, Greenberg A. J Biol Chem. 1999;274:7–10. doi: 10.1074/jbc.274.1.7. [DOI] [PubMed] [Google Scholar]
- 22.Yussman MG, Toyokawa T, Odley A, Lynch RA, Wu G, Colbert MC, Aronow BJ, Lorenz JN, Dorn GW. Nat Med. 2002;8:725–730. doi: 10.1038/nm719. [DOI] [PubMed] [Google Scholar]
- 23.Jurgensmeier JM, Xie Z, Deveraux Q, Ellerby L, Bredesen D, Reed JC. Proc Natl Acad Sci USA. 1998;95:4997–5002. doi: 10.1073/pnas.95.9.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clarke SJ, McStay GP, Halestrap AP. J Biol Chem. 2002;277:34793–34799. doi: 10.1074/jbc.M202191200. [DOI] [PubMed] [Google Scholar]
- 25.Bouillet P, Strasser A. J Cell Sci. 2002;115:1567–1574. doi: 10.1242/jcs.115.8.1567. [DOI] [PubMed] [Google Scholar]
- 26.Sowter HM, Ratcliffe PJ, Watson P, Greenberg AH, Harris AL. Cancer Res. 2001;61:6669–6673. [PubMed] [Google Scholar]
- 27.Galvez AS, Brunskill EW, Marreez Y, Benner BJ, Regula KM, Kirschenbaum LA, Dorn GW. J Biol Chem. 2006;281:1442–1448. doi: 10.1074/jbc.M509056200. [DOI] [PubMed] [Google Scholar]
- 28.Dooley TP, Miranda M, Jones NC, DePamphilis ML. Development (Cambridge, UK) 1989;107:945–956. doi: 10.1242/dev.107.4.945. [DOI] [PubMed] [Google Scholar]
- 29.Kina T, Ikuta K, Takayama E, Wada K, Majumdar AS, Weissman IL, Katsura Y. Br J Haematol. 2000;109:280–287. doi: 10.1046/j.1365-2141.2000.02037.x. [DOI] [PubMed] [Google Scholar]
- 30.Socolovsky M, Nam H, Fleming MD, Haase VH, Brugnara C, Lodish HF. Blood. 2001;98:3261–3273. doi: 10.1182/blood.v98.12.3261. [DOI] [PubMed] [Google Scholar]
- 31.Ikuta K, Weissman IL. Proc Natl Acad Sci USA. 1992;89:1502–1506. doi: 10.1073/pnas.89.4.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goodell MA, Rosenzweig M, Kim H, Marks DF, DeMaria M, Paradis G, Grupp SA, Sieff CA, Mulligan RC, Johnson RP. Nat Med. 1997;3:1337–1345. doi: 10.1038/nm1297-1337. [DOI] [PubMed] [Google Scholar]
- 33.Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Kontgen F, Adams JM, Strasser A. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 34.Bouillet P, Purton JF, Godfrey DI, Zhang LC, Coultas L, Puthalakath H, Pellegrini M, Cory S, Adams JM, Strasser A. Nature. 2002;415:922–926. doi: 10.1038/415922a. [DOI] [PubMed] [Google Scholar]
- 35.Ranger AM, Zha J, Harada H, Datta SR, Danial NN, Gilmore AP, Kutok JL, Le Beau MM, Greenberg ME, Korsmeyer SJ. Proc Natl Acad Sci USA. 2003;100:9324–9329. doi: 10.1073/pnas.1533446100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Testa U, Fossati C, Samoggia P, Masciulli R, Mariani G, Hassan HJ, Sposi NM, Guerriero R, Rosato V, Gabbianelli M, et al. Blood. 1996;88:3391–3406. [PubMed] [Google Scholar]
- 37.Pecci A, Travaglino E, Klersy C, Invernizzi R. Acta Haematol. 2003;109:29–34. doi: 10.1159/000067275. [DOI] [PubMed] [Google Scholar]
- 38.De Maria R, Testa U, Luchetti L, Zeuner A, Stassi G, Pelosi E, Riccioni R, Felli N, Samoggia P, Peschle C. Blood. 1999;93:796–803. [PubMed] [Google Scholar]
- 39.Gregory T, Yu C, Ma A, Orkin SH, Blobel GA, Weiss MJ. Blood. 1999;94:87–96. [PubMed] [Google Scholar]
- 40.De Maria R, Zeuner A, Eramo A, Domenichelli C, Bonci D, Grignani F, Srinivasula SM, Alnemri ES, Testa U, Peschle C. Nature. 1999;401:489–493. doi: 10.1038/46809. [DOI] [PubMed] [Google Scholar]
- 41.Negoro S, Oh H, Tone E, Kunisada K, Fujio Y, Walsh K, Kishimoto T, Yamauchi-Takihara K. Circulation. 2001;103:555–561. doi: 10.1161/01.cir.103.4.555. [DOI] [PubMed] [Google Scholar]
- 42.Chen G, Prchal JT. Best Pract Res Clin Haematol. 2006;19:387–397. doi: 10.1016/j.beha.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 43.Spivak JL. Blood. 2002;100:4272–4290. doi: 10.1182/blood-2001-12-0349. [DOI] [PubMed] [Google Scholar]
- 44.Coultas L, Bouillet P, Loveland KL, Meachem S, Perlman H, Adams JM, Strasser A. EMBO J. 2005;24:3963–3973. doi: 10.1038/sj.emboj.7600857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hutcheson J, Scatizzi JC, Bickel E, Brown NJ, Bouillet P, Strasser A, Perlman H. J Exp Med. 2005;201:1949–1960. doi: 10.1084/jem.20041484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Farley FW, Soriano P, Steffen LS, Dymecki SM. Genesis. 2000;28:106–110. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.