Abstract
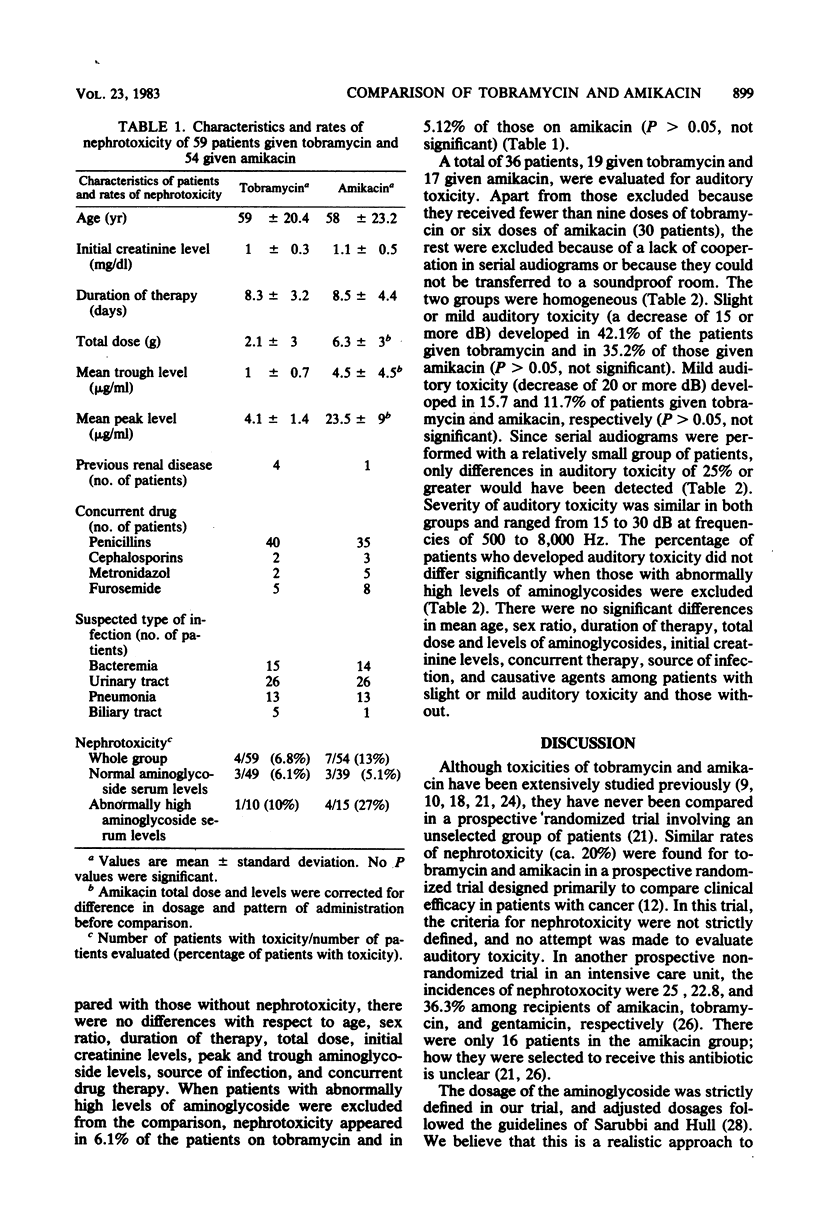
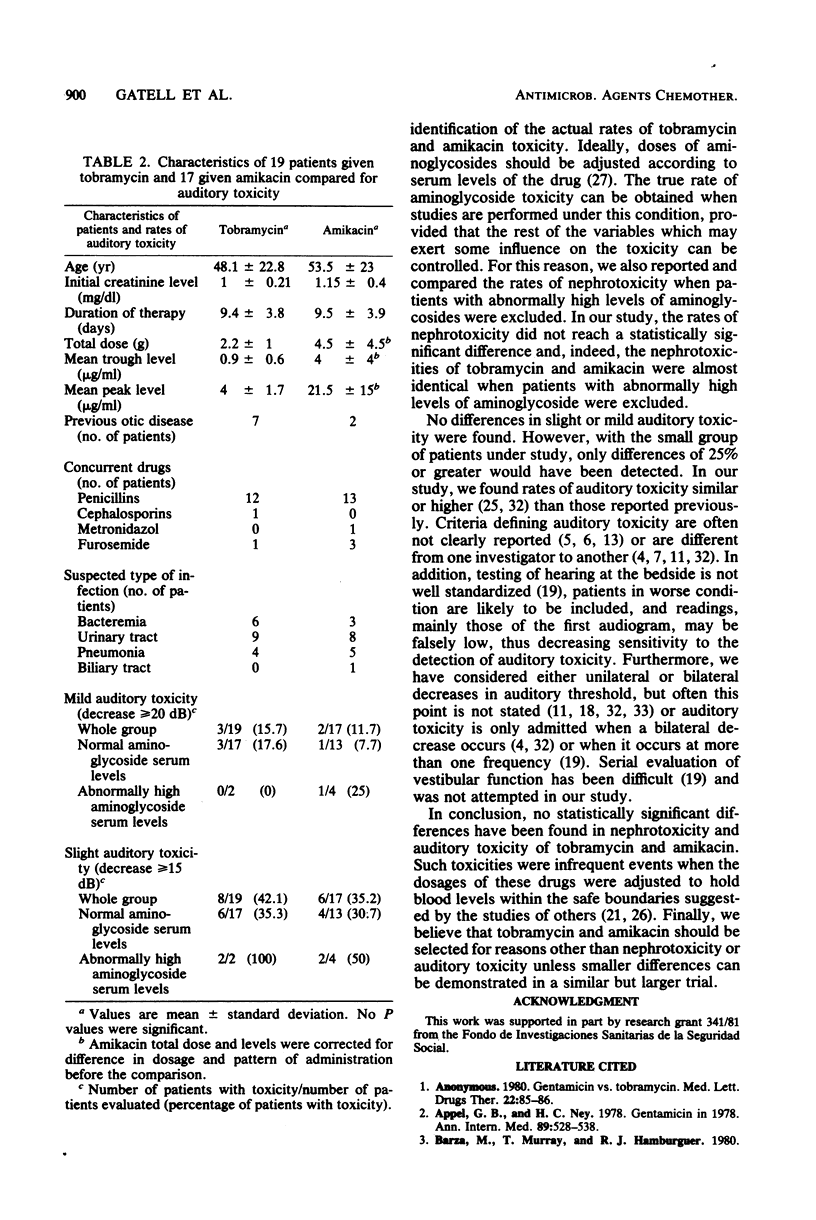
A total of 157 patients were treated with tobramycin or amikacin in a controlled prospective randomized trial. Dosages were adjusted to renal function according to a nomogram. Trough and peak aminoglycoside levels were available at the end of the trial. Of the above total, 113 recipients of nine or more doses of tobramycin or six or more doses of amikacin, without other apparent cause of renal failure, were evaluated for nephrotoxicity. Thirty-six patients were evaluated for auditory toxicity. The patients in groups evaluated for either nephrotoxicity or auditory toxicity were similar with respect to intensity and etiology of bacterial disease, concurrent exposure to other antimicrobial drugs, age and sex distribution, initial serum creatinine level, and total dose and duration of antimicrobial therapy. Nephrotoxicity of similar severity developed in 4 of 59 (6.8%) recipients of tobramycin and in 7 of 54 (13.1%) recipients of amikacin (P greater than 0.05). Mild auditory toxicity developed in 3 of 19 (15.7%) recipients of tobramycin and in 2 of 17 (11.7%) recipients of amikacin (P greater than 0.05). When patients with abnormally high mean trough or peak aminoglycoside levels were excluded from comparison, nephrotoxicity was 6.12 and 5.12% (P greater than 0.05) and auditory toxicity was 17.6 and 7.69% (P greater than 0.05) in the groups given tobramycin and amikacin, respectively. We conclude that the nephrotoxicity and auditory toxicity of amikacin and tobramycin are not significantly different and that such toxicities are indeed infrequent events when the dosages of these drugs are adjusted to hold blood levels within the safe boundaries suggested by the studies of others.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appel G. B., Neu H. C. Gentamicin in 1978. Ann Intern Med. 1978 Oct;89(4):528–538. doi: 10.7326/0003-4819-89-4-528. [DOI] [PubMed] [Google Scholar]
- Barza M., Murray T., Hamburger R. J. Uptake of gentamicin by separated, viable renal tubules from rabbits. J Infect Dis. 1980 Apr;141(4):510–517. doi: 10.1093/infdis/141.4.510. [DOI] [PubMed] [Google Scholar]
- Bender J. F., Fortner C. L., Schimpff S. C., Grove W. R., Hahn D. M., Love L. J., Wiernik P. H. Comparative auditory toxicity of aminoglycoside antibiotics in leukopenic patients. Am J Hosp Pharm. 1979 Aug;36(8):1083–1087. [PubMed] [Google Scholar]
- Bendush C. L., Weber R. Tobramycin sulfate: a summary of worldwide experience from clinical trials. J Infect Dis. 1976 Aug;134 (Suppl):S219–S234. doi: 10.1093/infdis/134.supplement_1.s219. [DOI] [PubMed] [Google Scholar]
- Black R. E., Lau W. K., Weinstein R. J., Young L. S., Hewitt W. L. Ototoxicity of amikacin. Antimicrob Agents Chemother. 1976 Jun;9(6):956–961. doi: 10.1128/aac.9.6.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera J., Arroyo V., Ballesta A. M., Rimola A., Gual J., Elena M., Rodes J. Aminoglycoside nephrotoxicity in cirrhosis. Value of urinary beta 2-microglobulin to discriminate functional renal failure from acute tubular damage. Gastroenterology. 1982 Jan;82(1):97–105. [PubMed] [Google Scholar]
- Coca A., Blade J., Martinez A., Segura F., Soriano E., Ribas-Mundo M. Tobramycin nephrotoxicity. A prospective clinical study. Postgrad Med J. 1979 Nov;55(649):791–796. doi: 10.1136/pgmj.55.649.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fee W. E., Jr Evaluation clinique de l'ototoxicité des aminosides. Comparaison de la tobramycine et de la gentamicine. Rapport préliminaire. Nouv Presse Med. 1978 Nov 29;7(42):3854–3855. [PubMed] [Google Scholar]
- Gooding P. G., Berman E., Lane A. Z., Agre K. A review of results of clinical trials with amikacin. J Infect Dis. 1976 Nov;134(Suppl):S441–S445. doi: 10.1093/infdis/135.supplement_2.s441. [DOI] [PubMed] [Google Scholar]
- Kumin G. D. Clinical nephrotoxicity of tobramycin and gentamicin. A prospective study. JAMA. 1980 Oct 17;244(16):1808–1810. [PubMed] [Google Scholar]
- Lane A. Z., Wright G. E., Blair D. C. Ototoxicity and nephrotoxicity of amikacin: an overview of phase II and phase III experience in the United States. Am J Med. 1977 Jun;62(6):911–918. doi: 10.1016/0002-9343(77)90660-x. [DOI] [PubMed] [Google Scholar]
- Lerner S. A., Seligsohn R., Matz G. J. Comparative clinical studies of ototoxicity and nephrotoxicity of amikacin and gentamicin. Am J Med. 1977 Jun;62(6):919–923. doi: 10.1016/0002-9343(77)90661-1. [DOI] [PubMed] [Google Scholar]
- Luft F. C., Bloch R., Sloan R. S., Yum M. N., Costello R., Maxwell D. R. Comparative nephrotoxicity of aminoglycoside antibiotics in rats. J Infect Dis. 1978 Oct;138(4):541–545. doi: 10.1093/infdis/138.4.541. [DOI] [PubMed] [Google Scholar]
- Meyer R. D. Amikacin. Ann Intern Med. 1981 Sep;95(3):328–332. doi: 10.7326/0003-4819-95-3-328. [DOI] [PubMed] [Google Scholar]
- Meyer R. D., Lewis R. P., Finegold S. M. Amikacin therapy for gram-negative septicemia. Am J Med. 1977 Jun;62(6):930–935. doi: 10.1016/0002-9343(77)90663-5. [DOI] [PubMed] [Google Scholar]
- Meyer R. D., Lewis R. P., Halter J., White M. Gentamicin-resistant Pseudomonas aeruginosa and Serratia marcescens in a general hospital. Lancet. 1976 Mar 13;1(7959):580–583. doi: 10.1016/s0140-6736(76)90370-6. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Bendush C. L. Ototoxicity of tobramycin: a clinical overview. J Infect Dis. 1976 Aug;134 (Suppl):S206–S218. doi: 10.1093/infdis/134.supplement_1.s206. [DOI] [PubMed] [Google Scholar]
- Plaut M. E., Schentag J. J., Jusko W. J. Aminoglycoside nephrotoxicity: comparative assessment in critically ill patients. J Med. 1979;10(4):257–266. [PubMed] [Google Scholar]
- Reymann M. T., Bradac J. A., Cobbs C. G., Dismukes W. E. Correlation of aminoglycoside dosages with serum concentrations during therapy of serious gram-negative bacillary disease. Antimicrob Agents Chemother. 1979 Sep;16(3):353–361. doi: 10.1128/aac.16.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarubbi F. A., Jr, Hull J. H. Amikacin serum concentrations: prediction of levels and dosage guidelines. Ann Intern Med. 1978 Nov;89(5 Pt 1):612–618. doi: 10.7326/0003-4819-89-5-612. [DOI] [PubMed] [Google Scholar]
- Schentag J. J., Plaut M. E., Cerra F. B. Comparative nephrotoxicity of gentamicin and tobramycin: pharmacokinetic and clinical studies in 201 patients. Antimicrob Agents Chemother. 1981 May;19(5):859–866. doi: 10.1128/aac.19.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklaver A. R., Greenman R. L., Hoffman T. A. Amikacin therapy of gram-negative bacteremia and meningitis. Treatment in diseases due to multiple resistant bacilli. Arch Intern Med. 1978 May;138(5):713–716. [PubMed] [Google Scholar]
- Smith C. R., Baughman K. L., Edwards C. Q., Rogers J. F., Lietman P. S. Controlled comparison of amikacin and gentamicin. N Engl J Med. 1977 Feb 17;296(7):349–353. doi: 10.1056/NEJM197702172960701. [DOI] [PubMed] [Google Scholar]
- Tally F. P., Louie T. J., Weinstein W. M., Bartlett J. G., Gorbach S. L. Amikacin therapy for severe gram-negative sepsis. Emphasis on infections with gentamicin-resistant organisms. Ann Intern Med. 1975 Oct;83(4):484–488. doi: 10.7326/0003-4819-83-4-484. [DOI] [PubMed] [Google Scholar]
- Walker B. D., Gentry L. O. A randomized, comparative study of tobramycin and gentamicin in treatment of acute urinary tract infections. J Infect Dis. 1976 Aug;134 (Suppl):S146–S149. doi: 10.1093/infdis/134.supplement_1.s146. [DOI] [PubMed] [Google Scholar]


