Abstract
A natural infection with Helicobacter pylori (H. pylori) in domestic cats (Felis cattus) less than 2 years of age has been well described in a closed colony of animals. Six cats from this colony that were serially evaluated by culture, polymerase chain reaction, and light and electron microscopy for a period of 3 years demonstrated persistent gastric colonization with a single cag− vac+ strain of H. pylori. In these cats, as well as five other 5- to 6-year-old cats that were examined, a long-term infection resulted in chronic diffuse lymphofollicular atrophic gastritis with areas of mucosal dysplasia in the antrum and predominantly midsuperficial gastritis in the body and cardia. Topographically, the distribution of lesions was similar in both young and older cats and closely resembled that found in humans, with the most severe changes occurring in the gastric antrum. Few granulocytes and no significant elevation in mast cells were seen in older H. pylori-infected cats compared with uninfected controls; however, marked increases in interepithelial globule leukocytes and numerous active mucosal lymphoid follicles were present in infected animals. Indices of gastritis were significantly greater in older infected cats when compared with uninfected controls and younger cats (P < 0.05). The antral cell proliferation index of infected older cats was significantly (P = 0.021) greater than that of uninfected controls. Apoptotic indices of the gastric antrum and body of infected cats were significantly (P = 0.01) increased versus controls. Chronic infection with H. pylori in cats shares many features of long-term H. pylori infection in humans, including the development of preneoplastic processes. This similarity provides useful, comparative insights into host-pathogen interactions.
Helicobacter pylori (H. pylori) is recognized as the primary cause of chronic gastritis, gastric and duodenal ulcers, and gastric cancer in humans. 1 Despite the emergence of H. pylori as a significant human pathogen, the pathogenesis of associated gastric disease is not fully elucidated; animal models are being used to study host-pathogen interaction and to help understand the complex and multifaceted disease process elicited by the bacterium. 2
Experimental models of H. pylori infection have been described in several species including gnotobiotic piglets, germ-free dogs, rats, gerbils, and mice. 2 The gastritis produced by H. pylori in most of these models demonstrates features of the lymphocytic inflammation observed in children, rather than chronic active gastritis with neutrophil infiltration seen in adult humans with a long-term infection. 3 The Mongolian gerbil is a promising model for experimental H. pylori infection. After being infected with H. pylori, gerbils initially develop a polymorphonuclear gastritis that can progress to gastric ulcers, preneoplastic lesions, and in some studies gastric adenocarcinoma. 4 The H. pylori-mouse model is increasingly being used for vaccine studies, as well as studies in pathogenesis. 5 These models have been helpful in studying selected mechanisms that are involved in the pathogenesis of gastric disease and possible therapeutic interventions. However, the coevolutionary interrelationship between host immunity, pathogen virulence factors, and the resultant disease expression has been difficult to assess, because none of the models are naturally occurring diseases in animals.
A natural H. pylori infection has been found only in the domestic cat and selected macaque species. 6 Nonhuman primates also have been used as an experimental model of H. pylori infection. 7,8 In nonhuman primates, coinfection with Helicobacter heilmannii can be a confounding factor in the interpretation of gastric pathology and disease mechanisms elicited by H. pylori. 9 Whereas gastric colonization with several Helicobacter species, including H. felis and H. heilmannii, 2,10 in domestic cats is common, a colony of cats naturally infected with H. pylori, but devoid of large gastric spiral organisms has been identified. 11 Although pet cats have not been found to harbor H. pylori despite the high prevalence of large gastric spirals, 12,13 H. pylori readily colonizes the cat stomach and can subsequently be detected in feline saliva and feces. 11,14 The goals of this study were to assess whether naturally infected cats remain persistently colonized with H. pylori, to characterize H. pylori-associated gastric pathology in cats on a longitudinal basis, and to compare findings with those encountered in chronic H. pylori infections in humans.
Materials and Methods
Animals
Six domestic shorthaired cats (three neutered males, three females) 6 to 7 months of age (Table 1 ▶ , group A) and five female cats 5 to 6 years of age (Table 1 ▶ , group B) were obtained from a commercial source and were known to be infected with H. pylori and free of retroviral, ie, feline leukemia virus (FELV) or feline immunodeficiency virus (FIV), infection. The younger cats were sequentially monitored by gastric endoscopy and biopsy analysis for a 3-year period. Four additional female cats, aged 6 to 7 years (Table 1 ▶ , group C), were obtained from a different vendor and were previously determined to be free of gastric Helicobacter organisms. 14 Cats were housed in animal facilities accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care. The six younger cats were pair housed in stainless steel cages. Short-term individual housing in stainless steel cages was provided for the older animals. Each cage was equipped with objects and cage furniture adequate to enrich the animal’s environment. To minimize any potential microbial cross-contamination, H. pylori-free cats were housed in a separate building. The temperature (68–74°F) and humidity (40–60%) were controlled; lights were maintained at 12 hours of light/12 hours of dark, and there were 10 to 15 air changes per hour in the animal rooms. All cats were examined daily and given food (Iams Cat Diet, Iams Co., Dayton, OH) and water ad libitum.
Table 1.
Number (N) and Composition of the Groups of Cats Examined
| Group | Composition | N |
|---|---|---|
| A | H. pylori+ serially sampled cats from ages 8 months to 3 years | 6 (3M, 3 F) |
| B | H. pylori+ cats 5–6 years of age | 5 (F) |
| C | H. pylori− cats 6–7 years of age | 4 (F) |
| D | H. pylori+ cats 1 year of age | 6 (M) |
M, Male; F, Female.
Specimen Collection
Gastric juice was retrieved by aspiration from the stomach via orogastric intubation using either 3.5 or 5.0 French polypropylene tubes (Sovereign catheters, Sherwood Medical, St. Louis, MO) introduced under ketamine (Ketaset, Fort Dodge Animal Health, Fort Dodge, IA) sedation. Mucosal gastric biopsies were obtained from the antrum, body, and cardia of all cats during endoscopy (group A) or at necropsy (groups B and C). Biopsy forceps and a 4.9-mm Pentax pediatric bronchoscope were used to retrieve 2-mm pinch biopsies from the stomach using ketamine/halothane (Halocarbon Laboratories, River Edge, NJ) anesthesia. Vomitus was collected from animals that vomited on recovery from anesthesia.
Older cats (groups B and C) were euthanized with an intravenous injection of euthanasia solution (Henry Schein, Melville, NY) containing sodium pentobarbital. Animals euthanized for complete postmortem evaluation had samples from all portions of the stomach frozen at −70°C for polymerase chain reaction (PCR) analysis.
Culture of H. pylori
All samples were suspended in 1.0 ml of Brucella broth (Difco Laboratories, Detroit, MI) with 15% glycerol and processed within an hour of collection. Gastric biopsy samples were homogenized before plating on selective media, consisting of blood agar base #2 (Difco), supplemented with 5% horse blood and antibiotics: 50 μg/ml amphotericin B, 100 μg/ml vancomycin, 3.3 μg/ml polymixin B, 200 μg/ml bacitracin, and 10.7 μg/ml nalidixic acid (Sigma Chemical Co., St. Louis, MO). 5 Culture plates were placed in vented jars, which were evacuated in 507 to 570 mm Hg and refilled to atmospheric pressure with an N2, H2, and CO2 mixture (80:10:10). The jars were then incubated for 3 weeks at 37°C and checked weekly for growth. Bacteria were identified as H. pylori by Gram stain; morphology under phase microscopy; oxidase, catalase, and urease reactions; resistance to nalidixic acid; and susceptibility to cephalothin.
DNA Extraction and PCR
DNA was extracted from both gastric tissue samples and gastric isolates by using the High Pure PCR Template Preparation Kit (Boehringer Mannheim, Indianapolis, IN). DNA preparation (10 μl) was added to a 100-μl reaction mixture containing 1× Taq polymerase (2.25 mmol/L MgCl2), 200 μmol/L of each deoxynucleotide, 0.5 μmol/L of each primer, and 200 μg/ml of bovine serum albumin. Helicobacter genus-specific and Helicobacter species-specific primers were used, including primers for H. pylori, H. heilmannii, H. felis, and H. bilis. PCR reactions were performed as follows: denaturation at 94°C for 1 minute, annealing at 58°C for 2 minutes, and extension at 72°C for 2 minutes. Thirty-five cycles were used for amplification in a MiniCycler PTC-150 (MJ Research, Inc., Watertown, MA).
To assess the homogeneity of bacterial isolates from different cats, restriction fragment length polymorphism (RFLP) analysis was performed. For RFLP analysis, the primer sequences chosen for amplification were specific for the H. pylori flaA gene. These two oligonucleotides, 5′ATG GCT TTT CAG GTC AAT AC3′ and 5′GCT TAA GAT ATT TTG TTG AAC G3′, produced a product of 1.5 kb. Amplified DNA (20 μl) was digested with 10 U of enzyme in the appropriate buffer recommended by the enzyme manufacturer at 37°C for 3 hours. Restriction enzyme patterns for HhaI and HaeIII were compared after the digested PCR products were run on 3% agarose gels. 14
Histopathology
Complete postmortem evaluations of nine 5- to 7-year-old animals (groups B and C) were performed. Fresh tissue samples of stomach, intestine, and selected visceral organs were immersion-fixed in 10% buffered formalin, paraffin-embedded, sectioned at 6 μm, and stained with 1) hematoxylin and eosin for morphological evaluation, 2) Warthin-Starry silver method for in situ detection of bacteria, 3) toluidine blue stain for detection of mast cells, or 4) Alcian blue/periodic acid-Schiff (PAS) reaction, pH 2.5, 15 or the high-iron diamine technique for mucin staining and histochemical evaluation of metaplasia. Duodenal colonization of H. pylori in areas of the mucosa with the gastric phenotype was assessed by staining with PAS, followed by prolonged (10 minute) staining with hematoxylin. 16 The surface area of the duodenal mucosal epithelium with a gastric mucin phenotype was determined morphometrically in the proximal 11 mm of the duodenum, beginning at the pyloric-duodenal junction, by importing images of PAS-stained sections into the Optimas 6.1 image software (Media Cybernetics, Silver Spring, MD) and recording the linear surface area of PAS-positive epithelium in the mucosa, in both control and infected animals.
Gastric biopsy samples taken from younger cats (group A) at 22, 38, and 43 months of age were stained with hematoxylin and eosin for morphological evaluation and the Warthin-Starry silver method for in situ detection of bacteria. Additionally, archival formalin-fixed stomach tissue, taken at necropsy from the greater curvature of six male, 1-year-old cats (Table 1 ▶ , group D) naturally infected with H. pylori 11 , was stained with hematoxylin and eosin, as well as with the Warthin-Starry silver method.
Electron Microscopy
A section of antrum from the H. pylori-infected older cats was fixed at necropsy in 2% glutaraldehyde and 0.1 mol/L sodium cacodylate buffer, pH 7.4. Tissues were postfixed in 1% osmium tetroxide, en bloc-stained with 2% aqueous uranyl acetate, dehydrated in graded alcohols and propylene oxide, and then embedded in LX112 resin (Ted Pella, Inc., Redding, CA). Thin sections were cut on an Ultracut E ultramicotome (Leica Microsystems, Inc., Deerfield, IL), mounted on formvar- and carbon-coated grids, and examined in a JEOL 100CX electron microscope (JEOL USA, Inc., Peabody, MA).
Topographic Scoring of Gastritis
Stomachs were systematically, microscopically evaluated at sites along the greater and lesser curvatures and at the cardia and proximal duodenum. Stomach lesions were scored semiquantitatively, based on the nature and topography of the inflammatory changes, and they were compared with findings in humans with H. pylori infection 17 (Figure 1) ▶ . The severity of gastritis was scored semiquantitatively using the method published by Genta et al. 17,18 Briefly, 8-mm sections of gastric mucosa from 5- to 7-year-old cats (groups B and C) were assessed for the extent and type of inflammatory cell infiltrates (neutrophils, eosinophils, mononuclear leukocytes). The density of H. pylori colonization was also determined within each of these sections. Additionally, mucosal mast cells, globule leukocytes, and lymphoid follicles were quantified within these 8-mm sections. Semiquantitative inflammatory scores, H. pylori density, and the number of lymphoid follicles were similarly determined for 8-mm gastric tissue sections of archival specimens taken from the greater curvature of 1-year-old H. pylori-infected cats 11 (group D) to assess the progression of H. pylori-induced lesions. These indices were compared with those obtained from the equivalent gastric sites on the greater curvature of infected 5- to 6-year-old cats (group B) at necropsy.
Figure 1.
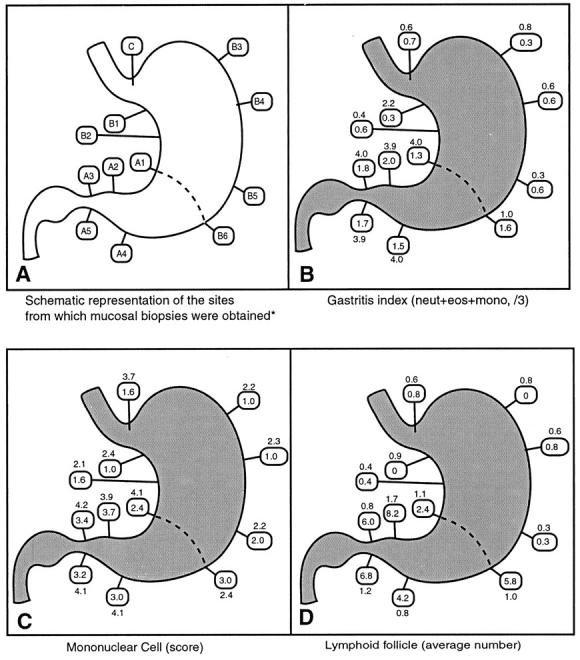
A−D: Schematic representations of source of biopsy materials (A). Topographic distribution of gastritis indices (B), mononuclear cell scores (C), and average number of lymphoid follicles (D) in the stomach of 5- to 6-year-old (group B) H. pylori-infected cats (see boxes). Indices for humans are outside the boxes. *Adapted from Reference 17.
Inflammatory scores obtained from 2-mm gastric biopsies taken from serially sampled younger H. pylori-infected cats (group A) were analyzed by gender and gastric location.
Immunohistochemistry of B and T Cells
Stomach samples from the antra and bodies of older H. pylori-infected cats (group B) were additionally characterized phenotypically with the anti-CD45RA antibody for identification of feline B cells, and the anti-CD3 antibody to identify T lymphocytes. Tissue sections were rehydrated for the CD3 antibody; tissues were blocked with Peroxoblock (Zymed Laboratories, Inc., San Francisco, CA) for 20 minutes and rinsed with phosphate-buffered saline (PBS), incubated in 200 U pronase/50 ml PBS for 10 minutes, and rinsed extensively with water followed by PBS. For CD45RA detection, staining was done as previously described. 19 A primary antibody (anti-CD3 or anti-CD79α; Dako Corp., Carpinteria, CA) was applied at a 1:100 dilution. Sections were washed with PBS, and a secondary antibody (biotinylated goat anti-rabbit IgG or rabbit anti-mouse IgG; Zymed) was applied at a 1:100 dilution for 30 minutes. Sections were again rinsed thoroughly with PBS, and streptavidin peroxidase was applied for 30 minutes. Sections were then rinsed in PBS, and staining was visualized with diaminobenzidine (DAB) or aminoethyl carbazole chromogens.
Apoptosis Staining and Scoring
The terminal deoxy-nucleotidyl-transferase-mediated dUTP-biotin nick end-labeling method (ApopTag, Oncor, Gaithersburg, MD) was used to stain cells in formalin-fixed feline gastric tissue and rat mammary gland control slides, based on the manufacturer’s directions. Staining was visualized with DAB chromogen, and apoptosis was assessed in conjunction with cell morphology. Apoptosis of the epithelium was quantified by counting the number of positive cells within a gastric pit. Positive-staining interepithelial lymphocytes were not counted. A mean number of 10 well-oriented gastric pits was examined for each specimen obtained from the five older H. pylori-infected cats (group B) and the four age-matched controls (group C). Specimens included sections taken from the lesser curvature of the body and from the greater curvature of the antrum. The labeling index was expressed as the number of positive-staining cells within 10 gastric pits and was calculated for both stomach antrum and body.
Bromodeoxyuridine Immunohistochemistry
Four 5- to 6-year-old, H. pylori-infected female cats from group B and four age-matched uninfected controls (group C) were evaluated using bromodeoxyuridine (BrdU) immunocytochemistry. Each cat was administered a single 100-mg/kg injection of BrdU solution via intravenous catheter, using a 22-mm filter. Stock solution (50 mg/ml) was prepared immediately before injection by dissolving BrdU (Sigma) in sterile water for injection (Abbott Laboratories, North Chicago, IL). The animals were euthanized and necropsied 4.5 hours after receiving the BrdU injection. Stomach tissue samples were then fixed in Carnoy’s solution and paraffin embedded.
After deparaffinization, the endogenous peroxidase activity was blocked by incubating tissues at room temperature with Peroxoblot (Zymed) for 1 minute and then rinsing them in PBS three times. The control samples were similarly handled. The slides were then incubated in 1 mol/L HCl at 60°C for 8 minutes to denature the tissue DNA. The slides were subsequently incubated with a monoclonal antibody to BrdU (Dako), diluted 1:40 in Tris-buffered saline (TBS), followed by incubation with biotinylated anti-mouse IgG (Dako;1:200 in TBS) and peroxidase-conjugated streptavidin (1:400 in TBS). The labeled cells were visualized with DAB chromogen and lightly counterstained with hematoxylin. The slides were washed with TBS between each incubation step.
Four entire tissue sections were examined for each cat. Specimens included sections taken from the lesser curvature of the body and from the greater curvature of the antrum. Only gastric pits longitudinally sectioned within the upper third of gastric mucosa and visible in their entire length were analyzed. A mean number of 10 well-oriented gastric pits was examined for each specimen. The labeling index was measured by counting the number of BrdU-positive-staining cells 20 and expressed as the result of the number of positive-staining cells within 10 gastric pits. The labeling index was calculated for both the stomach antrum and body.
Statistical Analyses
Nonparametric tests were used to analyze the data. Mann-Whitney U tests were used when inflammatory indices from each gastric region were compared between groups of infected and uninfected cats, as well as between infected cats of different ages. Comparisons within the groups between the indices from each gastric region were done by using Wilcoxon sign rank tests. Inflammatory indices were generated for individual older cats (groups B and C) by calculating the average score for specimens obtained from each of five sites from the antrum and six sites from the body. Only one site was evaluated from the cardia (Figure 1 ▶ , schematic diagram). The total numbers of mast cells, globule leukocytes, and lymphoid follicles counted were averaged for each gastric location. When comparing tissue samples from 5- to 6-year-old infected cats (group B) with archival specimens of 1-year-old infected cats (group D), the average gastritis index, lymphoid follicle number, and H. pylori score from corresponding sites within each gastric location were used (two sites from the antrum and four from the body along the greater curvature). Again, only one site was evaluated and compared from the cardia of 1-year-old (group D) and 5- to 6-year-old (group B) infected cats. The page test for ordered alternatives 21 was used to assess whether H. pylori-induced inflammation increased over time in serially sampled cats (group A). Spearman-rank correlations were calculated if appropriate. The median, average, and standard error of the mean (SEM) are reported for each index. Significance was set at a level of P < 0.05. The Stata software package (Stata, Inc., College Station, TX) was used to perform the statistical analyses.
Results
Culture and PCR
Cultures of stomach biopsies from serially sampled younger cats (group A) examined at 8, 11, 22, 27, and 38 months of age demonstrated that all six cats had persistent gastric colonization with H. pylori. PCR analyses of two bacterial isolates from the stomachs of these cats were positive, using H. pylori species-specific primers. PCR analyses of stomach tissue biopsies from these six cats taken at 43 months of age were 6/6 (100%) positive, using H. pylori species-specific primers, and all were negative using H. felis species-specific primers. Gastric juice was positive by culture for H. pylori in 2/6 (34%) of samples analyzed at 27 months of age and 6/6 (100%) of samples at 28 months of age. Two of three (67%) serially sampled cats had positive H. pylori cultures from vomitus samples. The five older cats (group B) obtained from the same source were all positive for H. pylori by culture of gastric mucosa. Two of four (50%) had positive H. pylori cultures from gastric fluid.
The PCR analyses of the stomach tissue of these five older cats (group B) showed evidence of H. pylori infection. The PCR analyses of bacterial isolates from four of these cats were also positive, using all Helicobacter and H. pylori 16S ribosomal DNA-specific primers. All five cats were negative by PCR analyses of stomach tissue, using primers specific for H. heilmannii, H. felis, and H. bilis. Four aged-matched cats (group C) from an alternate commercial source were confirmed negative by stomach tissue culture and PCR analyses with H. pylori-, H. heilmanni-, and H. felis-specific primers.
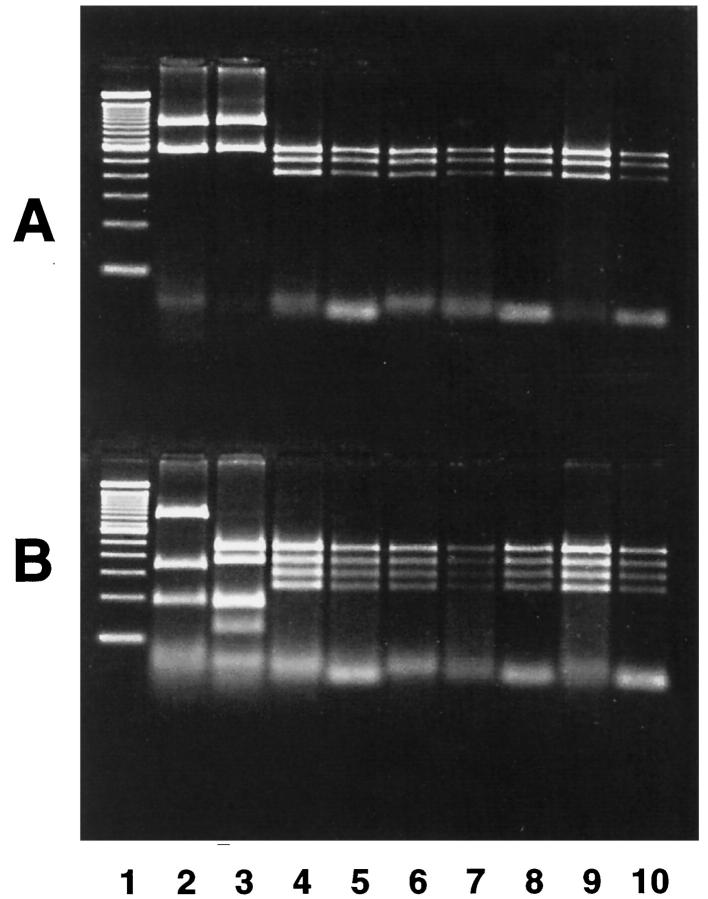
HhaI and HaeIII restriction enzyme patterns for the H. pylori flaA gene extracted from bacterial isolates from two serially sampled young cats from group A and four of the older cats from group B were the same (Figure 2) ▶ .
Figure 2.
RFLP profile of fla gene DNA extracted from cat and human H. pylori isolates. DNA was digested with HhaI (A) and HaeIII (B). Lanes: 1, 100-bp DNA ladder; 2, human H. pylori, Sidney strain; 3, human H. pylori, strain 264; 4, cat 10, age 5 years; 5, cat 7, age 5 years; 6, cat 8, age 6 years; 7, cat 9, age 6 years; 8, cat 1, age 38 months; 9, cat 2, age 38 months; 10, cat 1, age 22 months.
Pathology
At necropsy, the five older H. pylori-infected cats (group B) all had moderate to severe, multifocal or coalescent, irregular nodular thickening of the antral gastric mucosa. The nodular thickening was most pronounced in the distal antrum, although, in some areas of the proximal antrum, thickened areas were slightly raised and associated with partial loss of gastric folds. No gross lesions were present in the four age-matched control cats (group C).
The topography of inflammatory changes in older H. pylori-infected cats (group B) is shown in Figure 1 ▶ and compared with findings in humans with H. pylori-induced disease. 17 Gastric lesions consisted of inflammation with lymphoproliferation, atrophy, and glandular dysplasia and had similar topographical distributions with comparable magnitudes of severity in all of the chronically infected (group B) 5- to 6-year-old animals (Figure 3) ▶ . The antrum was most severely affected, with the highest levels of inflammation occurring along the lesser curvature; inflammation was diffuse throughout the full thickness of the mucosa and was composed of infiltrates of lymphocytes and plasma cells with fewer mononuclear leukocytes resembling macrophages and scattered neutrophils, eosinophils, and mast cells. Variably sized lymphoid aggregates and organized follicles, many with germinal centers, were also present in the deep to mid-antral mucosa. In addition, larger follicles, particularly near the pyloric-duodenal junction, extended from the muscularis mucosae to the superficial mucosa, replacing gastric pits and creating a dome-shaped lumenal surface lined by partially flattened epithelium. Occasionally, lymphoid follicles were present in the submucosa. Additional significant findings included increased numbers of interepithelial lymphocytes and globule leukocytes throughout the inflamed areas of the antrum but, most notably, above and adjacent to mucosal lymphoid aggregates and follicles (Figure 4) ▶ . Changes in the antral-mucosal epithelium included areas of mucin depletion; variable degrees of glandular atrophy with uniform or partial attenuation of epithelium; hyperplasia and dysplasia, often associated with lymphoepithelial lesions; and complete mucosal atrophy consisting of loss of glandular density and collapse of connective tissue in the lamina propria (Figure 3) ▶ .
Figure 3.
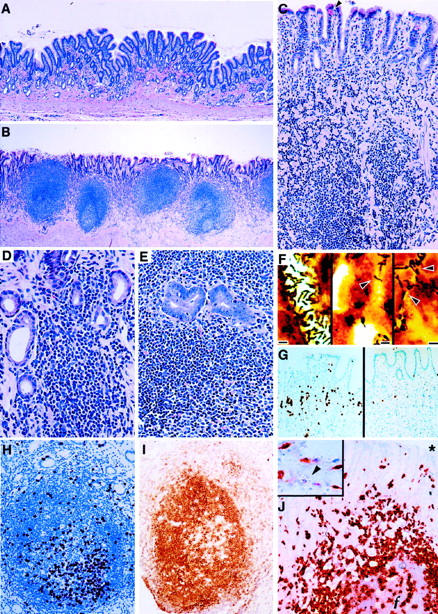
Photomicrographs from 5- to 6-year-old cats (group B) naturally infected with H. pylori and uninfected age-matched controls (group C). A: Gastric antrum of a 5-year-old Helicobacter-free control cat showing normal mucosa (H&E; original magnification, ×44). B: Characteristic lesions of lymphofollicular gastritis in the gastric antrum of H. pylori-infected cat (H&E; original magnification, ×38). C: Gastric antrum of H. pylori-infected cat showing diffuse lymphocytic infiltrates into the lamina propria and within the mucosal epithelium and severe atrophy with loss of glandular density and collapse of connective tissue. Interepithelial globule leukocytes are also present (arrowhead); (H&E; original magnification, ×85). D: Higher magnification of H. pylori-infected cat with progressive glandular destruction associated with infiltrates of lymphocytes (H&E; original magnification, ×165). E: Area of glandular dysplasia with lymphoepithelial infiltration in severely inflamed gastric antrum (H&E; original magnification, ×160). F: Colonization of gastric antrum (left), body (middle), and cardia (right) by homogeneous populations of helical bacteria typical of H. pylori (arrowheads) (scale bars = 3 μm, Warthing-Starry; original magnification, ×480). G: Immunohistochemistry for BrdU uptake showing marked mucosal epithelial proliferation in the antrum of H. pylori-infected cat (left) compared with noninfected control (right) (DAB with hematoxylin counterstain; original magnification, ×64). H: typical active lymphoid follicle in the antrum of H. pylori-infected cat, showing lymphoproliferation with BrdU uptake (DAB with hematoxylin counterstain; original magnification, ×142). I: Immunostaining with anti-CD45RA for B lymphocytes in antral mucosal lymphoid follicle (DAB with hematoxylin counterstain; original magnification, ×145). J: Identification of T cell infiltrates with anti-CD3 staining. T cells are present throughout the lamina propria, within the mucosal epithelium, and at the periphery and center of a lymphoid follicle (ƒ); the asterisk (*) denotes the gastric lumen above the mucosa (3-amino-9-ethylcarbazole with hematoxylin counterstain; original magnification, ×150). J (inset): CD3-positive T cells in the gastric lamina propria and lack of CD3 staining of globule leukocyte (arrowhead) ; original magnification, ×213.
Figure 4.
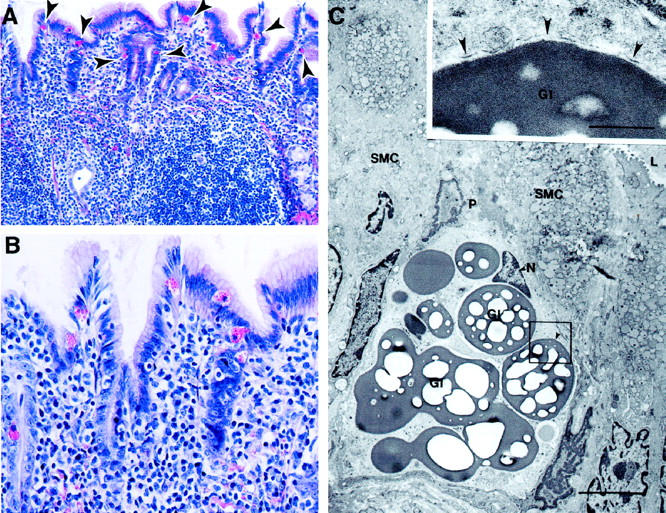
Photomicrographs from H. pylori-infected 5- to 6-year-old cats (group B). A: Chronic diffuse atrophic gastritis with extensive lymphocytic infiltrates associated with increased globule leukocytes in the overlying mucosal epithelium (arrowheads; H&E; original magnification, ×89). B: Higher magnification showing globule leukocytes with large eosinophilic cytoplasmic granules; also evident are numerous interepithelial lymphocytes (H&E; original magnification, ×183). C: Globule leukocyte within the gastric mucosa of an older H. pylori-infected cat. Globule leukocytes were characterized ultrastructurally by their large globular inclusions (GI) that consisted of a homogeneous electron dense interior (inset) with many electron lucent spots. Most of the circumference of each globular inclusion was surrounded by plasma membrane (inset, arrowheads). A pseudopod (P) in the globule leukocyte extends between surface mucous cells (SMC). The nucleus (N) of the globule leukocyte was small and located on the luminal side of the cell. L, lumen of a gastric gland. Low-magnification electron micrograph; original magnification, ×5560; scale bar, 5 μm. C (inset): Original magnification, ×27,800; scale bar, 1 μm.
In the gastric body, multifocal, superficial lymphocytic gastritis was present, often in conjunction with solitary lymphoid aggregates or follicles in the deep mucosa and adjacent infiltrates of predominantly mononuclear inflammatory cells in the lamina propria. Occasionally, foci of fundic mucosal-gland atrophy were evident in inflamed areas. The cardia similarly had superficial lymphocytic gastritis with few neutrophils and eosinophils, scattered mast cells, and occasional poorly organized lymphoid aggregates in the mid- to deep mucosa. Interepithelial globule leukocytes were increased in both the body and cardia of infected cats.
The Warthin-Starry-stained sections demonstrated helical bacteria characteristic of H. pylori in the antrum, body, and cardia of the five older infected cats (Figure 3 ▶ , group B). Bacteria were present in pits and lumina of glands throughout the full thickness of mucosa in the antrum and cardia, whereas colonization in the body was typically limited to the superficial mid-mucosa. The presence of helical bacteria in the mucosa was invariably associated with local inflammation. H. pylori was not seen in the cytoplasm of cells or in the lamina propria, and no other morphotypes of bacteria were seen in any sections of the stomach. In the duodenum of chronically infected cats, rare helical bacteria resembling H. pylori were seen apposed to the villous mucosal epithelium in Warthin-Starry-stained sections.
Staining of the stomach with Alcian blue, pH 2.5, and PAS or high-iron diamine did not demonstrate intestinal metaplasia in the stomach in infected (group B) or control animals (group C). However, PAS staining revealed the presence of gastric-type mucin expression in some areas of the lining epithelium and in glands of the proximal duodenum for both infected (group B) and control cats (group C). PAS-positive areas were most prominent in the proximal duodenum but were present in some animals up to 22 mm from the gastroduodenal junction. Morphometric analysis of the surface area of the PAS-positive duodenal epithelium did not demonstrate a significant difference between older control (group C) and infected (group B) cats.
Immunophenotypic analysis of stomachs from older infected cats (group B) demonstrated that diffuse lymphocytic infiltrates in the lamina propia and within the mucosal epithelium were predominantly CD3+ T cells, whereas lymphoid nodules and follicles were chiefly composed of CD45+ B cells with scattered T lymphocytes present in germinal centers. Follicular germinal centers also contained cells morphologically resembling dendritic cells and tingible body macrophages. Globule leukocytes in the stomach were CD3-negative. Immunohistochemical detection of BrdU labeling demonstrated markedly active lymphoid proliferation within most follicles (Figure 3) ▶ .
Endoscopic gastric biopsies taken from serially examined H. pylori-infected cats (group A) at 22, 38, and 43 months of age demonstrated chronic lymphocytic gastritis in the antrum, body, and cardia, similar to, although less severe than, gastritis in the 5- to 6-year-old infected cats (group B). Two of the animals from group A sampled at 43 months of age additionally had mucosal atrophy in the antrum. Warthin-Starry-positive helical bacteria characteristic of H. pylori were present in biopsy samples in all six (100%) group A animals at 22 months of age, 5 of 6 (83%) at 38 months of age, and 4 of 6 (67%) at 43 months of age. No other morphotypes of bacteria were seen in the stomach at any time in any animals.
Archival tissue specimens from 1-year-old H. pylori-infected cats 11 (group D) had lymphoplasmacytic follicular gastritis involving the antrum, body, and cardia. Lesions were similar to those seen in other age animals (groups A and B) but lacked significant atrophy and dysplasia. Warthin-Starry-stained sections demonstrated H. pylori organisms in all group D animals, and the presence of bacteria coincided with areas of inflammation.
Indices of Inflammation
The median, mean, and SEM are listed in Table 2 ▶ for indices of inflammation in 5- to 7-year-old cats (groups B and C) determined for the gastric antrum, body, and cardia. The gastritis index and the number of globule leukocytes were significantly greater in the antrum and body of older H. pylori-infected cats (group B) when compared with the corresponding sites of age-matched (group C) controls (P = 0.01). Mast cell numbers and distribution did not differ significantly between older infected (group B) and uninfected (group C) cats (data not shown). Both groups had a larger number of mast cells within the stomach body when compared with the antrum and cardia, but the number was only significantly greater in the body, 171.75 (mean ± SE, 169.41 ± 16.61), when compared with that of the antrum, 71.8 (mean ± SE, 80.47 ± 15.27; P = 0.043) in the H. pylori-infected cats (group B).
Table 2.
Gastritis Index, H. pylori Score, and Number of Lymphoid Follicles and Globular Leukocytes in the Antrum, Body, and Cardia of H. pylori-infected (Group B) and Noninfected (Group C) Older Cats
| Site | H. pylori | Gastritis index | Lymphoid follicles | Globule leukocytes | ||||
|---|---|---|---|---|---|---|---|---|
| Infected (group B) | Noninfected (group C) | Infected (group B) | Noninfected (group C) | Infected (group B) | Noninfected (group C) | Infected (group B) | Noninfected (group C) | |
| Antrum | 2.0 (2.04 ± 0.38); P = 0.011 | 0 | 1.67 (1.64 ± 0.07); P = 0.014 | 0.26 (0.24 ± 0.06) | 5.80 (5.60 ± 1.01); P = 0.015 | 0 | 18.67 (20.67 ± 3.24); P = 0.014 | 3.63 (4.25 ± 1.37) |
| Body | 1.67 (1.54 ± 0.17); P = 0.01 | 0 | 0.84 (0.80 ± 0.08); P = 0.012 | 0 (0.03 ± (0.03) | 1.67 (1.70 ± 0.23); P = 0.01 | 0 | 12.83 (14.18 ± 2.32); P = 0.014 | 2.67 (2.46 ± 0.74) |
| Cardia | 1.0 (1.40 ± 0.51); P = 0.05 | 0 | 0.67 (0.67 ± 0.18); NS | 1.0 (0.78 ± 0.40) | 0 (0.80 ± 0.58); NS | 0 | 3.0 (5.20 ± 2.27); NS | 2.0 (4.34 ± 2.33) |
Data in parentheses are means ± SEs. P values indicate significant differences between the median values of the two groups. NS, not significant.
Generally, the distribution of inflammatory indices corresponded with H. pylori colonization in the gastric mucosa. There was no significant correlation between the gastritis index and the H. pylori score in the antrum or body of infected older cats (group B). The gastritis index and number of lymphoid follicles of infected older cats (group B) were significantly greater in the antrum than in the body (P = 0.043). The antral gastritis index was also significantly greater than that of the cardia (P = 0.043), but the number of antral lymphoid follicles, although higher, was not significantly greater than that of the cardia, approaching significance only at P = 0.079. The number of globule leukocytes was significantly elevated in the antrum when compared with the body and cardia of older (group B) infected cats (P = 0.043). The number of globule leukocytes was significantly greater in the body than in the cardia of these group B cats (P = 0.043). In uninfected older cats (group C), the numbers of globule leukocytes found in the antrum, body, and cardia were not significantly different.
The gastritis indices calculated for biopsies of the antrum and body in serially examined younger cats (group A) at 22, 38, and 43 months of age were not significantly different. Biopsies of cardia of younger cats at 22 months of age were not obtained for histological analysis. The gastritis indices of male and female 3-year-old H. pylori-infected cats (group A) were not significantly different. Among the 3-year-old group A animals, the antral gastritis index of 0.67 (mean ± SE, 0.67 ± 0.07) was significantly greater than that of the body, 0.34 (mean ± SE, 0.37 ± 0.06; P = 0.003) and the cardia, 0.34 (mean ± SE, 0.42 ± 0.11; P = 0.013).
The gastritis index of the antrum in 5- to 6-year-old H. pylori-infected cats (group B) was significantly greater than that of 1-year-old (group D) infected cats (P = 0.006, Table 3 ▶ ). However, no significant differences were found between the two age groups in the antral H. pylori score and the number of lymphoid follicles. The gastritis index of the body was significantly greater in older (group B) cats (P = 0.01). The number of lymphoid follicles in the body of older cats (group B) was also greater than that of 1-year-old cats (group D), but this difference only approached significance at P = 0.065. Among the six 1-year-old group D infected animals, the antral gastritis index and number of lymphoid follicles were significantly greater than those of the body (P = 0.027), although the H. pylori score was not significantly different between antrum and body, similar to the older cats (group B).
Table 3.
Gastritis Index, H. pylori Score, and Number of Lymphoid Follicles Obtained from Greater Curvature Gastric Sites in H. pylori-infected 1-Year-Old (Group D) and 5- to 6-Year-Old (Group B) Cats
| Site | H. pylori | Gastritis index | Lymphoid follicles | |||
|---|---|---|---|---|---|---|
| 1 year old (group D) | 5–6 year old (group C) | 1 year old (group D) | 5–6 year old (group C) | 1 year old (group D) | 5–6 year old (group C) | |
| Antrum | 1.50 (1.50 ± 0.18); NS | 2.25 (2.25 ± 0.47) | 0.92 (0.95 ± 0.08); P = 0.006 | 1.51 (1.60 ± 0.11) | 7.0 (6.67 ± 1.01); NS | 5.0 (5.50 ± 1.12) |
| Body | 1.0 (1.11 ± 0.24); NS | 1.60 (1.62 ± 0.19) | 0.34 (0.32 ± 0.07); P = 0.01 | 0.84 (0.87 ± 0.13) | 0.17 (0.72 ± 0.56); NS | 2.25 (2.22 ± 0.31) |
| Cardia | 1.50 (1.50 ± 0.50); NS | 1.0 (1.20 ± 0.37) | 0.84 (0.84 ± 0.50); NS | 0.67 (0.67 ± 0.18) | 0.50 (0.50 ± 0.50); NS | 0 (0.80 ± 0.58) |
Data in parentheses are means ± SEs. P values indicate significant differences between the median values of the two age groups. NS, not significant.
BrdU Immunohistochemistry
None of the cats demonstrated any untoward reactions to the intravenous injection of the BrdU solution. The antral labeling index of infected older cats (group B) was greater than that of the uninfected controls (group C), 49.85 (mean ± SE, 53.25 ± 9.19) versus 20.10 (mean ± SE, 19.10 ± 1.59). This difference was statistically significant at P = 0.021 (Figure 5) ▶ . There was no significant difference between the labeling indices of the body of the older, infected (group B) and control (group C) cats.
Figure 5.
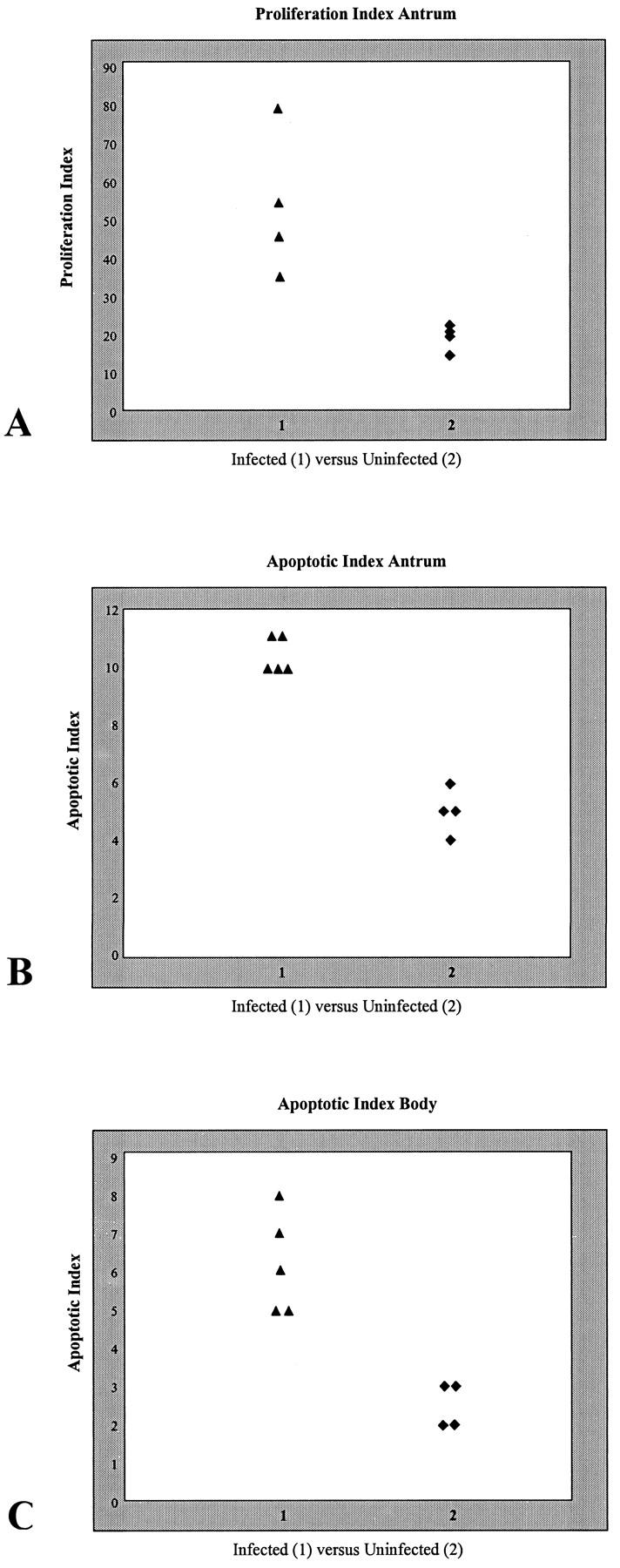
A: Proliferation index of the antrum of H. pylori-infected (group B) and uninfected (group C) older cats. B and C: Apoptotic indices of the antrum (B) and body (C) of H. pylori-infected (group B) and uninfected (group C) older cats.
Terminal Deoxynucleotidyl-Transferase-Mediated dUTP-Biotin Nick End-Labeling Histochemistry
The apoptotic index calculated for the antrum of infected older cats (group B) was significantly greater (P = 0.012) than that of uninfected controls (group C), 10 (mean ± SE, 10.40 ± 0.25) versus 5.0 (mean ± SE, 5.0 ± 0.41). The apoptotic index was also significantly elevated in the body (P = 0.013) of the infected older cats (group B), 6.0 (mean ± SE, 6.20 ± 0.58), when compared with that of the group C controls, 3.0 (mean ± SE, 2.67 ± 0.29; Figure 5 ▶ ).
Discussion
We have established that natural H. pylori infection in cats can persist and cause progressive gastritis, as also occurs in humans. The strain of H. pylori that persistently colonized all cats from this colony in our study was cag− vac+ (J Jones, personal communication). cag− status is consistent with the published findings in other cats from the same source. 14 Our findings using PCR with Helicobacter species-specific primers and the restriction enzyme digestion of the H. pylori fla gene, as well as the previous findings with RFLP analyses of H. pylori ureA and ureB genes in isolates from this closed colony, 22 confirm the lack of coinfection with other Helicobacter species and the genetic and biochemical homogeneity of H. pylori in these cats. In humans and rhesus monkeys, H. pylori is highly heterogeneous. 23 The homogeneity of H. pylori infection in this group of cats is advantageous in the study of the H. pylori pathogenesis and altered gastric-cell-proliferation kinetics by reducing confounding genetic variables.
In humans, H. pylori-associated gastritis can persist for years, and inflammation can progress to the development of ulcers and gastric cancer. 1 In H. pylori-infected cats, gastritis begins as early as 8 months; lesions consistently worsen with age and include the development of preneoplastic changes, despite a lack of alterations in other potential contributing factors, such as environmental stress and diet. Topographically, the distribution of lesions was similar in both young and older cats and closely resembled that found in humans, with the most severe changes occurring in the gastric antrum and the milder, although significant, lesions developing in the cardia and corpus. Despite similarities in the degree of colonization by H. pylori in different areas of the stomach, the intensity of antral inflammation exceeded that in other regions. This is also true of H. pylori in humans. 17 A significant difference in the topography of gastritis in cats and humans was seen in the cardia; in humans, the severity of inflammation in the cardia approximates that seen in the antrum, 17 whereas, in cats, lesions in the antrum were markedly more severe than those in the cardia. Although the reasons for this difference are unclear, further research defining possible host factors limiting inflammation in the cardia of infected cats may be useful in understanding human disease. An additional significant difference noted was the lack of ulcers in H. pylori-infected cats. Whether the absence of ulcerative disease in cats is related to infection with a cag− strain of H. pylori or results from unique features of the feline host immune response remains to be determined. Interestingly, no clear association has been found between the infection of pet cats with other helicobacter species, such as H. felis and H. heilmannii, and the development of either gastric or duodenal ulcers. 24
The nature of the inflammation in cats, although worsening over time, remained predominantly mononuclear throughout the study. In adult humans, H. pylori often elicits a considerable neutrophilic reaction, in addition to lymphocytic inflammation, 3 although the cag gene status of the infecting H. pylori strain(s) can affect the extent of neutrophil involvement. 25 In contrast, children develop lesions similar to those that we found in cats, with chiefly mononuclear leukocytic infiltrates and few granulocytes. 3 In cats, occasional neutrophils and eosinophils were present in most areas of gastritis. The cag− status of the H. pylori strain infecting cats may have contributed to the low level of granulocytic inflammation, although studies of experimental infection with cag+ strains in cats also reported lack of a significant neutrophil and eosinophil component. 14,26 The decreased granulocytic response may also have been related to a lack of involvement of other resident inflammatory cells, such as mucosal mast cells, or differences in cytokine profiles as lesions developed. In humans, mast cells have been implicated as potential immunomodulatory components of gastritis and may recruit neutrophils and eosinophils through production of interleukin-8 or interleukin-5, respectively. In one study, mast cells were shown to be increased in humans with H. pylori-associated gastritis. 27 An increase in mast cells has been recognized in several strains of mice associated with gastritis produced by experimental H. felis infection. 28 We found no significant changes in gastric mucosal mast cells in older H. pylori-infected cats when compared with the controls. Mast cell involvement in children has not been assessed.
An important comparative finding was the marked increase in globule leukocytes in older H. pylori-infected cats versus control animals. Globule leukocytes are migratory mononuclear cells that are found in the gastrointestinal tract of several species, including cats. 29 In goats, these cells are present within the epithelial mucosa of the small and large intestine and have been shown to be a γδ T-cell subset. 30 In cats, they are purported to be of large granular lymphocyte lineage, based on the demonstration of perforin-like substances within cytoplasmic granules, and they have a distribution very much like that of murine and ovine intestinal γδ T cells. 31 Perforin-like immunoreactivity suggests that feline globule leukocytes produce antimicrobial substances and support their role in the mucosal host defense. Globule leukocytes are increased in the stomachs of cats with nematode infestations and have also been reported in cheetahs with chronic gastritis caused by H. acinonyx. 32,33 In humans, globule leukocytes are an inflammatory component in chronic airway infections, including tracheitis and rhinitis, but have not been found in the gastrointestinal mucosa. 34,35 In our study, feline gastric globule leukocytes were CD3 negative, which, together with the previous demonstration of perforin-like immunoreactivity, 31 suggests that they may be lymphocytes of a natural killer (NK) cell lineage. NK cells have been shown to be increased in human patients with H. pylori-associated gastritis, although detailed studies of their role in the disease are lacking. 36 Similarly, the role of mucosal γδ T cells in humans after an infection with H. pylori is poorly understood. 37-39 The cat model of H. pylori gastritis offers a valuable and unique system for evaluating features of innate mucosal immunosurveillance in the gastric compartment by allowing assessment of both γδ T cells and globule leukocytes in host immunity.
Mucosal atrophy seen with chronic H. pylori infection is an important expression of disease that is usually linked with compensatory hyperplasia and dysplasia and is considered a precursor in the development of gastric adenocarcinoma. 40 Alterations in the balance of programmed cell death (apoptosis) and epithelial proliferation are central to the predisposition of cancer after gastritis. 41 In vivo studies with biopsy specimens from H. pylori-infected humans have demonstrated an increased rate of apoptosis in these patients. 42 Moreover, the eradication of H. pylori with antibiotic therapy results in a decreased rate of apoptosis. 42,43 The attachment of H. pylori to surface epithelial cells, and the activity of bacterial enzymes, including lipase, protease, and urease, as well as vacuolating cytotoxin produced by the bacteria, may result in cell injury and induction of apoptosis. 44 Inflammatory mediators produced during chronic infection, such as a reactive oxygen and nitrogen species, can also lead to increased apoptosis by damaging DNA. 45 The rate of cellular proliferation has likewise been shown to be higher in gastric epithelial cells of human patients with H. pylori gastritis. 42 Like humans, cats with chronic H. pylori infection develop significant mucosal atrophy as well as dysplasia in the antrum. These findings, along with the increases in both cellular proliferation and apoptosis in areas of severe chronic gastritis in older H. pylori-infected cats, are consistent with findings in humans and provide a suitable model for the serial investigation of progressive disease and the development of cancer.
Metaplasia is a well-recognized change in both the duodenum and stomach of people with chronic H. pylori infection. 40 In the duodenum, gastric metaplasia has been linked to gastric acid hypersecretion in patients with duodenal ulcers, 46 many of whom are infected with H. pylori. In cats, gastric metaplasia in the duodenum has been reported in association with a Helicobacter-like organism infection. 47 In our study, an expression of a gastric mucin phenotype was seen in the proximal duodenum of both H. pylori-infected and uninfected cats and was not associated with ulcerative lesions. Therefore, the interpretation that duodenal gastric metaplasia is a nonspecific response to mucosal injury by ingested irritants, among other environmental factors, 48 and not necessarily the result of an H. pylori infection, is supported by our findings in cats. We did not see intestinal metaplasia in the stomach of cats despite severe gastritis, unlike H. pylori disease in humans. This may be attributed to an age bias, because the oldest cats examined were middle-aged, or to differences in mucosal epithelial physiology.
Gastric neoplasia is among the leading causes of cancer-related mortality in people worldwide. In the United States, gastric carcinoma is one of the 20 most commonly diagnosed malignancies. 49 Gastric neoplasia has been well-documented in cats. Both adenocarcinoma and lymphoma have been reported. 50-52 Gastric mucosa-associated lymphoid tissue (MALT) lymphoma appears to have a higher prevalence in cats 24 and, in one case, was associated with H. heilmannii infection. 12 Gastric MALT lymphoma has also been documented in association with chronic H. heilmannii infection in humans. 53 The majority of alimentary lymphomas in cats are of B cell origin. 54,55 MALT lymphoma in human stomachs that develops with H. pylori-induced chronic inflammation demonstrates B cell monoclonality. 56 An important finding was the development of significant numbers of gastric lymphoid nodules and follicles in young cats (1–2 years old) which, by 5 to 6 years of age, exceeded levels seen in people with chronic H. pylori infection. These findings suggest that an initial stage of rapid folliculogenesis occurs in young cats and continues throughout the course of disease. The presence of mitotically active germinal centers in most of these follicles provides a nidus for subsequent development of gastric MALT lymphoma in cats. Feline leukemia virus has also been described as an etiology of alimentary lymphoma, including gastric lymphoma, in cats. 54 The potential for H. pylori, as well as other Helicobacter species, to act as a primary etiology or a cofactor, along with feline leukemia virus, in the development of gastric MALT lymphoma warrants further investigation.
In summary, use of naturally infected cats as a model of chronic H. pylori-induced disease in humans offers several advantages. The comparative physiology of immune cell reactivity and mucosal responses to chronic injury offer a valuable system for evaluating the molecular mechanisms of disease progression and the methods of therapeutic intervention in an animal model colonized with a single, well-characterized strain of H. pylori. The similarity to chronic human disease encountered in the feline model of a long term natural H. pylori infection, including the development of chronic atrophic gastritis, and increased levels of proliferation and apoptosis, make the cat a suitable model for study of these aspects of H. pylori pathogenesis.
Acknowledgments
We thank Dr. Melanie Ihrig (Massachusetts Institute of Technology Division of Comparative Medicine) for assistance with statistical analyses.
Footnotes
Address reprint requests to James G. Fox, MIT, Division of Comparative Medicine, 77 Massachusetts Avenue, Bldg. 16-825, Cambridge, MA 02139. E-mail: jgfox@mit.edu.
Supported by National Institutes of Health research grant R01-AI37750.
References
- 1.Graham DY: Helicobacter pylori infection in the pathogenesis of duodenal ulcer, and gastric cancer: a model. Gastroenterology 1997, 113:1983-1991 [DOI] [PubMed] [Google Scholar]
- 2.Fox JG, Lee A: The role of Helicobacter species in newly recognized gastrointestinal tract diseases of animals. Lab Anim Sci 1997, 47:222-255 [PubMed] [Google Scholar]
- 3.Lee A: Animal models for host-pathogen interaction studies. Br Med Bull 1998, 54:163-173 [DOI] [PubMed] [Google Scholar]
- 4.Honda S, Fujioka T, Tokeida M, Satoh R, Nishizono A, Nasu M: Development of Helicobacter pylori-induced gastric carcinoma in Mongolian gerbils. Cancer Res 1998, 58:4255-4259 [PubMed] [Google Scholar]
- 5.Lee A, O’Rourke J, De Ungria MC, Robertson B, Daskalopoulos G, Dixon MF: A standardized mouse model of Helicobacter pylori infection: introducing the Sidney strain. Gastroenterology 1997, 112:1386-1397 [DOI] [PubMed] [Google Scholar]
- 6.Dubois A: Animal models of Helicobacter infection. Lab Anim Sci 1998, 48:596-603 [PubMed] [Google Scholar]
- 7.Dubois A, Berg DE, Incecik ET, Fiala N, Heman-Ackah LM, del Valle J, Yang M, Wirth HP, Perez-Perez GI, Blaser MJ: Host specificity of Helicobacter pylori strains and host responses in experimentally challenged non-human primates. Gastroenterology 1999, 116:90-96 [DOI] [PubMed] [Google Scholar]
- 8.Dubois A, Lee CK, Fiala N, Kleanthous H, Mehlman PT, Monath T: Immunization against natural Helicobacter pylori infection in non-human primates. Infect Immun 1998, 66:4340-4346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reindel JF, Fitzgerald AL, Breider MA, Gough AW, Yan C, Mysore JV, Dubois A: An epizootic of lymphoplasmacytic gastritis attributed to Helicobacter pylori infection in cynomolgus monkeys (Macaca fascicularis). Vet Pathol 1999, 36:1-13 [DOI] [PubMed] [Google Scholar]
- 10.Otto G, Hazell SH, Fox JG, Howlett CR, Murphy JC, O’Rourke JL, Lee A: Animal and public health implications of gastric colonization of cats by Helicobacter-like organisms. J Clin Microbiol 1994, 32:1043-1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox JG, Perkins S, Yan L, Shen Z, Attardo L, Pappo J: Local immune response in Helicobacter pylori-infected cats and identification of H. pylori in saliva, gastric fluid and faeces. Immunology 1996, 88:400–406 [DOI] [PMC free article] [PubMed]
- 12.Neiger R, Dieterich C, Burnens A, Waldvogel A, Corthesy-Theulaz I, Halter F, Lauterburg B, Schassmann A: Detection and prevalence of Helicobacter infection in pet cats. J Clin Microbiol 1998, 36:634-637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Norris CR, Marks SL, Eaton KA, Torabian SZ, Munn RJ, Solnick JV: Healthy cats are commonly colonized with “Helicobacter heilmannii” that is associated with minimal gastritis. J Clin Microbiol 1999, 37:189-194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perkins SE, Fox JG, Marini RP, Shen Z, Dangler CA, Ge Z: Experimental infection in cats with a cagA+ human isolate of Helicobacter pylori. Helicobacter 1998, 3:225-235 [DOI] [PubMed] [Google Scholar]
- 15.Lev R, Spicer SS: Specific staining of sulfate groups with alcian blue at low pH. J Histochem Cytochem 1964, 12:309. [DOI] [PubMed] [Google Scholar]
- 16.Tazawa K, Tsutsumi Y: Effect of prolonged staining with hematoxilin on detecting Helicobacter pylori in hematoxilin-eosin-stained gastric mucosa. Pathol Int 1998, 48:448-452 [DOI] [PubMed] [Google Scholar]
- 17.Genta RM, Huberman RM, Graham DY: The gastric cardia in Helicobacter pylori infection. Hum Pathol 1994, 25:915-919 [DOI] [PubMed] [Google Scholar]
- 18.Genta RM, Lew GM, Graham DY: Changes in the gastric mucosa following eradication of Helicobacter pylori. Mod Pathol 1993, 6:281-289 [PubMed] [Google Scholar]
- 19.Jackson ML, Wood SL, Misra V, Haines DM: Immunohistochemical identification of B and T lymphocytes in formalin-fixed, paraffin-embedded feline lymphosarcomas: relation to feline leukemia virus status, tumor site, and patient age. Can J Vet Res 1996, 60:199-204 [PMC free article] [PubMed] [Google Scholar]
- 20.Fox JG, Li X, Cahill RJ, Andrutis K, Rustgi AK, Odze R, Wang TC: Hypertrophic gastropathy in Helicobacter felis-infected wild type C57BL/6 and p53 hemizygous transgenic mice. Gastroenterology 1996, 110:155-166 [DOI] [PubMed] [Google Scholar]
- 21.Siegel S, Castellan Jr NJ: Nonparametric Statistics for the Behavioral Sciences. New York, MacGraw-Hill, 1988, pp 184–188
- 22.Handt LK, Fox JG, Stalis IH, Rufo R, Lee G, Linn J, Li X, Kleanthous H: Characterization of feline Helicobacter pylori strains and associated gastritis in a colony of domestic cats. J Clin Microbiol 1995, 33:2280-2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drazek ES, Dubois A, Holmes RK: Characterization and presumptive identification of Helicobacter pylori isolates from rhesus monkeys. J Clin Microbiol 1994, 32:1799-1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jergens AE: Gastrointestinal disease and its management. Vet Clin North Am Small Anim Pract 1997, 27:1373-1402 [DOI] [PubMed] [Google Scholar]
- 25.Akopyants NS, Clifton SW, Kersulyte D, Crabtree JE, Youree BE, Reece CA, Bukanov NO, Drazek ES, Roe BA, Berg DE: Analyses of the cag pathogenicity island of Helicobacter pylori. Mol Microbiol 1998, 28:37-53 [DOI] [PubMed] [Google Scholar]
- 26.Fox JG, Batchelder M, Marini R, Yan L, Handt L, Li X, Shames B, Hayward A, Campbell J, Murphy JC: Helicobacter pylori-induced gastritis in the domestic cat. Infect Immun 1995, 63:2674-2681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakajima S, Krishnan B, Ota H, Segura AM, Hattori T, Graham DY, Genta RM: Mast cell involvement in gastritis with or without Helicobacter pylori infection. Gastroenterology 1997, 113:746-754 [DOI] [PubMed] [Google Scholar]
- 28.Mohammadi M, Redline R, Nedrud J, Czinn S: Role of the host in pathogenesis of Helicobacter-associated gastritis: H. felis infection of inbred and congenic mouse strains. Infect Immun 1996, 64:238–245 [DOI] [PMC free article] [PubMed]
- 29.Takeuchi A, Jervis HL, Sprinz H: The globule leukocyte in the intestinal mucosa in the cat: a histochemical, light and electron microscopy study. Anat Rec 1969, 164:79-99 [DOI] [PubMed] [Google Scholar]
- 30.Konno A, Hashimoto Y, Kon Y, Okada K, Davis WC, Sugimura M: Expression of gamma delta T cell receptor on caprine globule leukocytes. Vet Immunol Immunopathol 1995, 48:105-112 [DOI] [PubMed] [Google Scholar]
- 31.Konno A, Hashimoto Y, Kon Y, Sugimura M: Perforin-like immunoreactivity in feline globule leukocytes and their distribution. J Vet Med Sci 1994, 56:1101-1105 [DOI] [PubMed] [Google Scholar]
- 32.Hargis AM, Prieur DJ, Blanchard JL: Prevalence, lesions, and differential diagnosis of Ollulanus tricuspis infection in cats. Vet Pathol 1983, 20:71-79 [DOI] [PubMed] [Google Scholar]
- 33.Eaton KA, Radin MJ, Kramer L, Wack R, Sherding R, Krakowka S, Fox JG, Morgan DR: Epizootic gastritis associated with gastric spiral bacilli in cheetahs (Acinonyx jubatus). Vet Pathol 1993, 30:55-63 [DOI] [PubMed] [Google Scholar]
- 34.Baert J, Frederix M: Globule leukocytes in the respiratory epithelium of human upper airways: an ultrastructural study. Anat Rec 1985, 212:143-152 [DOI] [PubMed] [Google Scholar]
- 35.Austin LL, Dobbins WO: Intrepithelial leukocytes of the intestinal mucosa in normal man and in Whipple’s disease. Dig Dis Sci 1982, 27:311-320 [DOI] [PubMed] [Google Scholar]
- 36.Agnihotri N, Bhasin DK, Vohra H, Ray P, Singh K, Ganguly NK: Characterization of lymphocytic subsets and cytokine production in gastric biopsy samples from Helicobacter pylori patients. Scand J Gastroenterol 1998, 33:704-709 [DOI] [PubMed] [Google Scholar]
- 37.Engstrand L, Scheynius A, Pahlson C: An increased number of γδ T-cells and gastric epithelial cell expression of the groEL stress-protein homologue in Helicobacter pylori-associated chronic gastritis of the antrum. Am J Gastroenterol 1991, 86:976-980 [PubMed] [Google Scholar]
- 38.Trejdosiewicz LK, Calabrese A, Smart CJ, Oakes DJ, Howdle PD, Crabtree JE, Losowsky MS, Lancaster F, Boylston AW: γδ T cell receptor-positive cells of the human gastrointestinal mucosa: occurrence and V region gene expression in Helicobacter pylori-associated gastritis, coeliac disease and inflammatory bowel disease. Clin Exp Immunol 1991, 84:440-444 [PMC free article] [PubMed] [Google Scholar]
- 39.Seifarth C, Funk A, Reich K, Dahne I, Classen M, Deusch K: Selective increase of CD4+ and CD25+ T cells but not of γδ T cells in H. pylori associated gastritis. Advances in Mucosal Immunology. Edited by J Mestecky. New York, Plenum Press, 1995, pp 931–934 [PubMed]
- 40.Correa P: Helicobacter pylori and gastric carcinogenesis. Am J Surg Pathol 1995, 19(Suppl 1):S37-S43 [PubMed] [Google Scholar]
- 41.Moss SM: Review article: cellular markers in the gastric precancerous process. Aliment Pharmacol Ther 1998, 12(Suppl 1):91-109 [DOI] [PubMed] [Google Scholar]
- 42.Jones NL, Shannon PT, Cutz E, Yeger H, Sherman PM: Increase in proliferation and apoptosis of gastric epithelial cells early in the natural history of Helicobacter pylori infection. Am J Pathol 1997, 151:1695-1703 [PMC free article] [PubMed] [Google Scholar]
- 43.Hahm K, Lee KJ, Kim YS, Kim JH, Cho SW, Yim H, Joo HJ: Augmented eradication rates of Helicobacter pylori by new combination therapy with lansoprazole, amoxicillin, and rebamipide. Dig Dis Sci 1998, 43:235-240 [DOI] [PubMed] [Google Scholar]
- 44.Smoot DT: How does Helicobacter pylori cause mucosal damage? Gastroenterology 1997, 113:S331-S334 [DOI] [PubMed] [Google Scholar]
- 45.Correa P, Miller MJS: Helicobacter pylori and gastric atrophy-cancer paradoxes. J Natl Cancer Inst 1995, 87:1731-1732 [DOI] [PubMed] [Google Scholar]
- 46.Lee A, Dixon MF, Danon SJ, Kuipers E, Megraud F, Larsson H, Mellgard B: Local acid production and Helicobacter pylori: a unifying hypothesis of gastroduodenal disease. Eur J Gastroenterol Hepatol 1995, 7:461-465 [PubMed] [Google Scholar]
- 47.Serna JH, Genta RM, Lichtenberger LM, Graham DY, El-Zaatari FAK: Invasive Helicobacter-like organisms in feline gastric mucosa. Helicobacter 1997, 2:40-43 [DOI] [PubMed] [Google Scholar]
- 48.Wyatt JI, Rathbone BJ, Sobala GM, Shallcross T, Heatley RV, Axon ATR, Dixon MF: Gastric epithelium in the duodenum: its association with Helicobacter pylori and inflammation. J Clin Pathol 1990, 43:981-986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Breuer T, Malaty HM, Graham DY: The epidemiology of H. pylori-associated gastroduodenal diseases. The Immunobiology of H. pylori: from Pathogenesis to Prevention. Edited by PB Ernst, P Michetti, PD Smith. Philadelphia, Lippincott-Raven, 1997, pp 1–14
- 50.Turk MAM, Gallina AM, Russell TS: Nonhematopoietic gastrointestinal neoplasia in cats: a retrospective study of 44 cases. Vet Pathol 1981, 18:614-620 [DOI] [PubMed] [Google Scholar]
- 51.Cribb AE: Feline gastrointestinal adenocarcinoma: a review and retrospective study. Can Vet J 1988, 29:709-712 [PMC free article] [PubMed] [Google Scholar]
- 52.Brodey RS: Alimentary tract neoplasms in the cat: a clinicopathologic survey of 46 cases. Am J Vet Res 1966, 27:74-80 [PubMed] [Google Scholar]
- 53.Morgner A, Lehn N, Andersen LP, Thiede C, Bennedsen M, Neubauer B, Miehlke S, Neubauer A, Stolte M, Bayerdoerffer E: Helicobacter heilmannii-associated primary gastric low-grade MALT lymphoma: complete remission after antibacterial treatment. Gastroenterology 1999, 116:A469. [DOI] [PubMed] [Google Scholar]
- 54.Mahony OM, Moore AS, Cotter SM, Engler SJ, Brown D, Penninck DG: Alimentary lymphoma in cats: 28 cases (1988–1993). J Am Vet Med Asso 1995, 207:1593-1598 [PubMed] [Google Scholar]
- 55.Holmberg CA, Manning JS, Osburn BI: Feline malignant lymphomas: comparison of morphologic and immunologic characteristics. Am J Vet Res 1976, 37:1455-1460 [PubMed] [Google Scholar]
- 56.Nakamura S, Aoyagi K, Furuse M, Suekane H, Matsumoto T, Yao T, Sakai Y, Fuchigami T, Yamamoto I, Tsuneyoshi M, Fujishima M: B-cell monoclonality precedes the development of gastric MALT lymphoma in Helicobacter pylori-associated chronic gastritis. Am J Pathol 1998, 152:1271-1279 [PMC free article] [PubMed] [Google Scholar]