Abstract
To investigate the roles of B7-1 and/or B7-2 co-stimulatory molecule in the development of graft arterial disease (GAD), major histocompatibility complex (MHC) class II-mismatched allograft hearts were transplanted into wild-type, B7-1−/−, B7-2−/−, or B7-1/B7-2−/− recipient mice. Grafts were explanted at 4 or 8 weeks and used for histological and immunohistochemical analyses, RNase protection assay, and flow cytometry of graft infiltrating cells. Grafts in wild-type recipients showed macrophage, recipient MHC class II, and B7 molecule co-localization by immunohistochemistry to GAD lesions. Flow cytometry revealed that CD11b(+) and MHC class II(+) graft infiltrating cells expressed B7-1 more than B7-2, whereas B7-2 expression was predominant in CD11b(−) cells at 4 and 8 weeks. GAD was significantly attenuated in the allografts in B7-1−/− and B7-1/B7-2−/− but not in B7-2−/− recipients compared to wild-type hosts. Interferon-γ mRNA levels were comparable in all graft combinations, whereas interleukin-4 mRNA levels decreased in grafts in B7-2 deficient hosts, but did not correlate with GAD attenuation. The findings indicate distinct roles for B7-1 and B7-2 co-stimulatory molecules in the development of GAD, potentially because of differential expression of B7-1 and B7-2 molecules on distinct stimulator and/or effector cell populations.
T cell responses play a crucial role in acute allograft rejection as well as in the development of graft arterial disease (GAD) in long-term transplants. 1 GAD is a fibroproliferative intimal-based lesion that results in graft ischemia and failure, and is the major long-term limitation to solid organ transplantation. 2 In alloimmune responses, antigen-specific T cells are primed by interaction with major histocompatibility antigen complex (MHC)-bearing antigen presenting cells (APC). Complete activation requires additional co-stimulation, 3,4 with B7-CD28 interactions providing the most important secondary signals. 5 The B7-1 (CD80) and B7-2 (CD86) co-stimulatory molecules are expressed on professional APC and engage CD28 on T cells to transmit positive co-stimulatory signals. 6-8 Conversely, B7 interaction with the high-affinity receptor cytotoxic T lymphocyte-associated antigen-4 (CTLA4) transmits negative signals into T cells. B7/CD28 ligation may also mediate T cell activation by modulating other co-stimulatory pathways, including CD40/CD40 ligand interaction. 9 T cell activation is hypothesized to cause secondary macrophage recruitment and activation which in turn will induce vascular smooth muscle cell proliferation, migration, and matrix production leading to the lesion formation seen in GAD. 2
We have recently shown that total allomismatched cardiac grafts survived indefinitely in B7-1/B7-2−/− recipient mice, whereas wild-type or B7-1/B7-2−/− grafts transplanted into wild-type recipients were acutely rejected. 10 Moreover, in long-term allografts from B7-1/B7-2−/− recipients 10 or recipients treated with chronic CTLA4Ig injection, 11 GAD was infrequent and mild, suggesting abrogation of both acute rejection and GAD by the interruption of recipient B7 co-stimulation. To date, the relative expression of B7-1 or B7-2 co-stimulatory molecules in chronic allografts has not been well characterized, and it remains uncertain whether the blockade or depletion of either or both B7 molecules is required to prevent GAD development.
To elucidate potential differential effects of B7-1 or B7-2 deficiency on the development of GAD, we studied mouse hearts transplanted heterotopically. 12,13 Total allomismatch does not routinely permit long-term allograft survival in wild-type or B7-1−/− recipients. 14 Therefore, MHC class II disparate allografts were placed in wild-type, B7-1−/−, B7-2−/−, or B7-1/B7-2−/− mice receiving transient anti-CD4 and anti-CD8 immunosuppression before transplantation. In this model, transplanted heart allografts routinely survive longer than 12 weeks and reliably develop GAD lesions by 8 to 12 weeks after transplant. 12,13 We evaluated the extent of parenchymal rejection and GAD in these allografts, as well as the expression of B7-1 and B7-2 by immunohistochemistry and flow cytometry. We also performed immunohistochemical staining for CD28, CD40, CD40 ligand, MHC class II, monocyte/macrophage markers F4/80 and CD11b, CD4, CD8, and CD45R/B220 to assess the expression of co-stimulatory molecules in long-term grafts, the distribution of professional APC, and the population of graft infiltrating cells. Because cytokines play important roles in the pathogenesis of GAD 13,15 and B7 co-stimulation may drive Th cell differentiation, 16,17 we analyzed cytokine expression in long-term grafts by RNase protection assay (RPA).
In this study, a predominant expression of B7-1 relative to B7-2 was observed on perivascular MHC class II(+), CD11b(+) cells in chronic-stage allografts. In addition, interruption of B7-1 co-stimulation resulted in a greater inhibitory effect on the development of GAD than did disruption of B7-2 co-stimulation.
Materials and Methods
Animals
Inbred male C57BL/6 (B/6, H-2b) mice were obtained from Taconic Farms, Inc. (Germantown, NY). B6.C-H-2bm12KhEg (bm12, H-2 bm12) mice, which are MHC class II-mismatched with B/6, were obtained from The Jackson Laboratory (Bar Harbor, ME). Generation of B7-1−/−, 18 B7-2−/−, or B7-1/B7-2−/− 19 mice has been described previously; animals were back-crossed to B/6 mice for more than seven generations. All mice were maintained in the Harvard Medical School animal facilities, allowed ad libitum access to food and acidified water, and used at 9 to 12 weeks of age. All experiments conformed to animal care protocols approved by the institutional review group.
Antibodies and Other Reagents
Anti-CD4 antibody (clone GK1.5) and anti-CD8 antibody (clone 2.43) for immunosuppression were prepared from serum-free supernatants in an artificial capillary system (Cellmax; Celluco Inc., Rockville, MD) using hybridoma clones (American Type Culture Collection, Germantown, MD), purified by ammonium sulfate precipitation and size-exclusion chromatography, and concentrated to 1.5 mg/ml. Purified anti-CD45R/B220 antibody (RA3-6B2), purified anti-CD11b antibody (M1/70), purified anti-CD16/CD32 antibody (Fc-block; clone 2.4G2), purified anti-CD28 antibody (37.51), purified anti-CD4 antibody (RM4-5), purified anti-CD8 antibody (53–6.7), fluorescein isothiocyanate (FITC)-conjugated anti-CD11b antibody (M1/70), FITC-conjugated anti-I-Ab antibody (25-9-17), FITC-conjugated isotype-matched IgG controls, biotinylated anti-B7-1 antibody (16-10A1), biotinylated anti-B7-2 antibody (GL1), biotinylated anti-CD40 antibody (3/23), biotinylated anti-CD40 ligand antibody (MR1), biotinylated anti-I-Ab antibody (25-9-17), biotinylated isotype-matched IgG controls, phycoerythrin-conjugated streptavidin, and the Riboquant multiprobe RPA kit (mCK-1 and mCK-5) were obtained from PharMingen (San Diego, CA). Anti-I-Ab antibody (25-9-17) recognizes the B/6 MHC class II molecule but not the mutant bm12 MHC class II protein (confirmed by staining of spleens from these two strains, not shown). Anti-F4/80 antibody was from Serotec (Oxford, UK). Mouse-adsorbed biotinylated anti-rat IgG (H+L) antibody and biotinylated anti-hamster IgG (H+L) antibody were from Vector Laboratories, Inc. (Burlingame, CA). Fast Red tablets (substrate for alkaline phosphatase) and hematoxylin solution were provided by Sigma Chemical Co. (St. Louis, MO). Trizol RNA extracting solution was from Life Technologies, Inc. (Grand Island, NY). Ficoll lymphocyte separation medium was from Organon Teknika Corp. (Durham, NC).
Heart Transplantation
Heterotopic heart transplantation was performed as previously described. 13,20 In brief, donor and recipient mice were anesthetized by inhalation of Metofane (Pittman-Moore, Mundelein, IL). Donor hearts were perfused with chilled, heparinized saline via the inferior vena cava and harvested after ligation of the vena cava and pulmonary veins. Donor heart aorta and pulmonary artery were anastomosed to the abdominal aorta and inferior vena cava of a recipient mouse, respectively, using microsurgical technique. Ischemic time during the surgical procedure was routinely 30 minutes, and initial graft survival was >90%. For histological evaluation at 8 weeks, wild-type (n = 11), B7-1−/− (n = 7), B7-2−/− (n = 7), or B7-1/B7-2−/− (n = 5) mice of B/6 background served as recipients for bm12 donor allografts. For flow cytometry of graft infiltrating cells, hearts of bm12 mice were implanted to B/6 wild-type recipients (n = 25) and harvested at 4 or 8 weeks. B/6 isografts (n = 3) were used for isogeneic controls for histological and immunohistochemical examination, and RPA.
Immunosuppression
Immunosuppression was provided by a brief course of treatment with anti-CD4 (GK1.5) and anti-CD8 (2.43) monoclonal antibodies, achieving reproducible indefinite graft survival. 12,13 These antibodies (each at 1.5 mg/ml) were administered intraperitoneally at 6, 3, and 1 day before transplantation in a combined volume of 0.2 ml with no subsequent treatment. 12,13
Histological Examination
The grafts were explanted at 4 or 8 weeks after transplant, and cut transversely into three specimens. The basal portion was fixed in 10% buffered formalin, embedded in paraffin, and 5-μm sections were stained with hematoxylin and eosin, or for elastic tissue. The mid-transverse section was frozen in optimal cutting temperature (O.C.T.) compound (Ames Co., Division of Miles Laboratories, Elkhart, IN) for immunohistochemistry (see below), and the apical transverse section was used for either flow cytometry analysis or RPA. The severity of parenchymal rejection and GAD was scored blindly by two independent observers (YF and RNM). Scores uniformly fell within a range of one grade for both observers, and were averaged. Parenchymal rejection was graded using a scale modified from the International Society for Heart and Lung Transplantation (0, no rejection; 1, mild interstitial or perivascular infiltrate without necrosis; 2, focal interstitial or perivascular infiltrate with necrosis; 3, multifocal interstitial or perivascular infiltrate with necrosis; and 4, widespread infiltrate with hemorrhage and/or vasculitis). GAD scores were calculated as described previously by averaging the scores of arteries assessed from multiple histological sections (0, no or minimal, ie, <10% vascular occlusion; 1, 10 to 25% occlusion; 2, 25 to 50% occlusion; 3, 50 to 75% occlusion; 4, 75 to 100% occlusion); typically, >10 arteries (epicardial to medium-sized penetrating arteries) were evaluated from each specimen. 13
RNase Protection Assay
Total RNA was prepared by guanidinium thiocyanate/phenol/chloroform/isoamyl alcohol isolation method using Trizol (Life Technologies, Inc.). Ten micrograms of total RNA from each sample was subjected to quantitative analysis of cytokine and chemokine mRNA expression by RPA. Multiprobe RPA kit mCK-1 which contained DNA templates for interleukin (IL)-4, IL-5, IL-10, IL-13, IL-15, IL-9, IL-2, IL-6, interferon (IFN)-γ, L32, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and mCK-5 which contained DNA templates for lymphotactin, regulated on activation, normal T cell expressed and presumably secreted (RANTES), eotaxin, macrophage inflammatory protein-1β (MIP-1β), MIP-1α, MIP-2, IFN-inducible protein-10, monocyte chemoattractant protein-1, T cell activation gene 3, L32, and GAPDH, were used in this analysis. RPA was performed following the manufacturer’s instructions. The volume integrations of mRNA-incorporated 32P-labeled probe fragments were analyzed by bioimaging analyzer (Molecular Dynamics, Sunnyvale, CA). Finally, volume integrations of the protected bands for cytokines and chemokines were normalized against the bands for the GAPDH housekeeping gene in the corresponding lanes.
Immunohistochemical Staining
Five-μm-thick frozen sections were fixed in 4% paraformaldehyde dissolved in phosphate-buffered saline (PBS) or in acetone for 5 minutes at 4°C. Acetone fixation was used on sections stained for B7-2, CD28, and CD40, because fixation in 4% paraformaldehyde/PBS impaired the antibody reactivity. To reduce nonspecific binding, sections were first incubated in PBS with 1% bovine serum albumin, and 10% normal goat or rabbit serum for 30 minutes at room temperature (RT); 10 μg/ml of Fc-block (anti-CD16/CD32 Ab) was also applied when biotinylated antibodies were used as first antibodies. To stain B220, CD11b, CD28, CD4, CD8, and the macrophage marker F4/80, sections were incubated with unlabeled primary antibodies (each at 1 to 10 μg/ml) for 90 minutes at RT, washed in PBS, and incubated with biotinylated secondary antibodies (each at 2.5 or 5 μg/ml) for 45 minutes at RT. To stain I-Ab (MHC II), B7-1, B7-2, CD40, and CD40 ligand, biotinylated antibodies against these molecules were incubated with each section for 90 minutes at RT (each at 5 or 10 μg/ml). Biotinylated isotype-matched antibodies at the same concentrations, and for the same duration served as antibody-staining controls. After washing in PBS, the sections were incubated with an alkaline phosphatase-conjugated avidin-biotin complex (Vectastain ABC-AP kit; Vector Laboratories, Inc.) and washed. Alkaline phosphatase activity was visualized by incubating in substrate solution (Fast Red; Sigma Chemical Co.). Sections were counterstained with hematoxylin solution (Sigma Chemical Co.).
Flow Cytometry of Graft Infiltrating Cells
Graft infiltrating cells were recovered from the allografts implanted to wild-type recipients at 4 or 8 weeks after transplant and used for flow cytometry, as previously described. 21 Typically, graft infiltrating cells were pooled from the apices of four to six allografts to achieve satisfactory cell numbers; histology from these pooled hearts confirmed that they showed comparable rejection and GAD scores. The heart grafts were minced with a sterile razor blade and rocked at 37°C for 2 hours in 10 ml of borate-buffered saline with 2% bovine serum albumin and 20 mg collagenase (Sigma Chemical Co.). Digested graft tissues were strained through a 70-μm nylon cell strainer (Becton Dickinson, Mountain View, CA). Red blood cells, dead lymphocytes, and cardiomyocytes were removed by centrifugation through Ficoll (Organon Teknika Corp.) for 20 minutes at 800 rpm. Recovered interface cells were washed twice in cold PBS and then incubated with anti-mouse CD16/CD32 antibody (Fc block) for 10 minutes on ice to reduce background staining. Thereafter, cell surface antigens were stained using FITC-conjugated anti-CD11b antibody, anti-I-Ab antibody, biotinylated anti-B7-1 antibody, anti-B7-2 antibody, or isotype-matched control IgG. After 45 minutes incubation on ice and two washes in cold PBS, the cells were incubated with CyC-conjugated streptavidin for 30 minutes on ice. The stained cells were washed twice in cold PBS and fixed in 4% paraformaldehyde in PBS for 10 minutes at 4°C. After a wash in cold PBS, the stained cells were analyzed by flow cytometry on a FACScan flow cytometer (Becton Dickinson) using CellQuest analyzing software (Macintosh).
Statistical Analysis
Values for histological grading and for relative gene expression of cytokines are expressed as means ± SEM and compared between groups, using analysis of variance followed by Fisher’s protected least significant difference (PLSD) post hoc test.
Results
B7-1 Co-Stimulation by Recipient APC Plays an Important Role in the Development of GAD in Long-Term Allografts
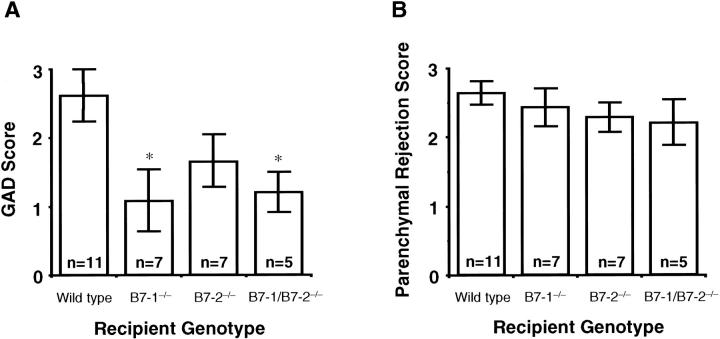
In control B/6 isografts, no GAD was observed at 8 weeks after transplant. In contrast, severe GAD developed in the bm12 to wild-type B/6 allografts by 8 weeks. The MHC class II disparate allografts implanted to B7-1/B7-2−/− or B7-1−/− recipients, showed significantly less severe GAD compared to wild-type recipients, whereas GAD did not differ significantly from wild-type in recipients lacking B7-2 (Figure 1 and 2A) ▶ ▶ .
Figure 1.
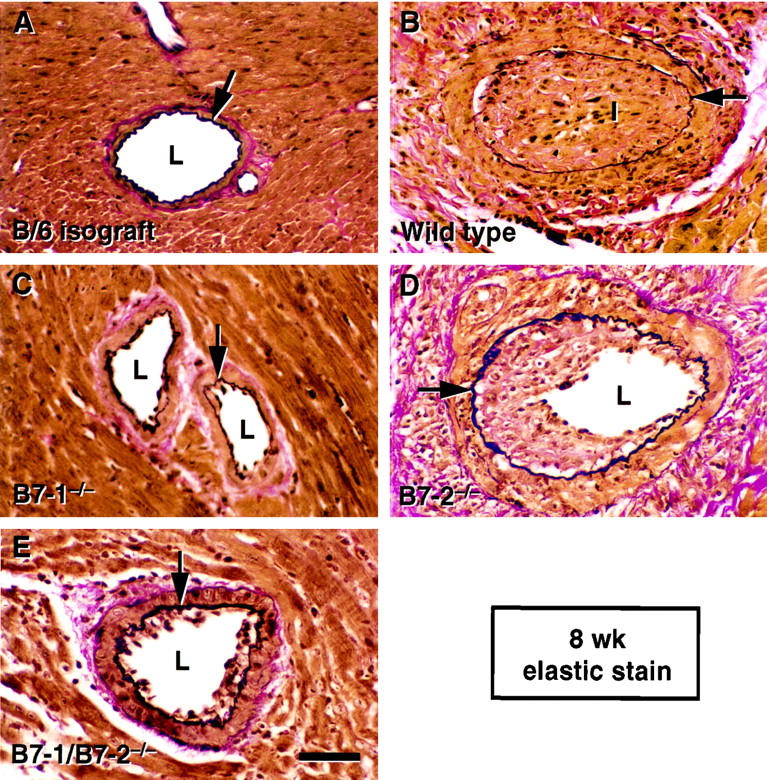
Elastic staining of the sections of mouse heart isograft and allografts at 8 weeks after transplant. Arrows indicate internal elastic laminae. L, lumen; I, intima. Scale bar = 50 μm. A: A representative section from a B/6 isograft. No GAD lesions developed in isografts by 8 weeks after transplant. B: A representative section from a bm12 to wild-type B/6 allograft shows severe intimal hyperplasia accompanied by perivascular accumulation of mononuclear graft infiltrating cells. C: A representative section from a bm12 to B7-1−/− B/6 allograft. GAD lesions that developed in the allografts implanted into B7-1−/− recipients were less severe than those in wild-type recipients. D: A representative section from a bm12 to B7-2−/− B/6 allograft. Results from multiple hearts showed no significant difference of severity in GAD between allografts in B7-2−/− B/6 recipients and wild-type recipients. E: A representative section from a bm12 to B7-1/B7-2−/− B/6 allograft. GAD lesions that developed in the allografts implanted into B7-1/B7-2−/− recipients were less severe than those in wild-type recipients. Mild GAD in a bm12 to B7-1/B7-2−/− B/6 allograft is shown in this panel, whereas most other vessels in this group did not show any histological evidence of GAD.
Figure 2.
A: Bar graphs showing the grading scores for GAD of allograft groups at 8 weeks after transplant. Wild-type bm12 allografts were transplanted to wild-type, B7-1−/−, B7-2−/−, or B7-1/B7-2−/− B/6 recipients. Values are expressed by mean ± SEM. *, P < 0.05 versus wild-type recipient group. B: Bar graphs showing the grading scores for parenchymal rejection of allograft groups at 8 weeks after transplant. Wild-type bm12 allografts were transplanted to wild-type, B7-1−/−, B7-2−/−, or B7-1/B7-2−/− B/6 recipients. Values are expressed by mean ± SEM.
Parenchymal Rejection Is Not Affected by the Absence of B7 Co-Stimulation in Long-Term Allografts
Histological evaluation indicated no significant differences in the severity of interstitial mononuclear cell infiltration between any recipient genotype groups at 8 weeks after transplantation (Figure 2B) ▶ . It should be emphasized that parenchymal rejection is denoted here as the extent of infiltrate and associated myocyte necrosis, and does not equate to graft failure (see Methods). No histological evidence of parenchymal rejection was observed in the isografts.
Cytokine and Chemokine Expression in the Allografts
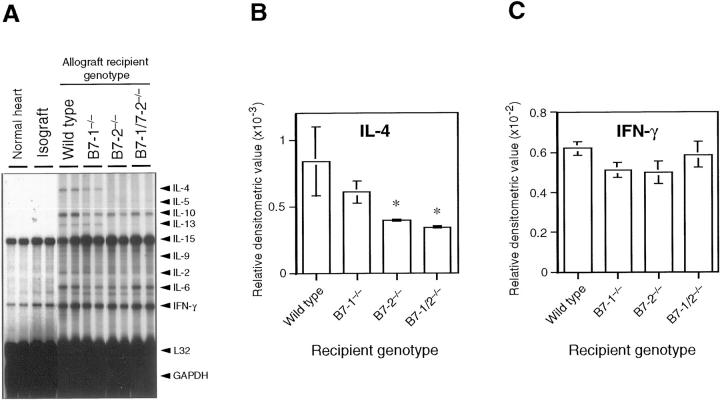
The Th1 cytokine IFN-γ and the Th2 cytokines IL-4, IL-10, and IL-13 were all detected in the allografts of the wild-type B/6 recipient group at 8 weeks. IL-4 expression was significantly less in grafts from the B7-2−/− and B7-1/B7-2−/− recipient groups, whereas IFN-γ, IL-6, and IL-10 expression were comparable among allografts from all allograft groups. IL-4 expression was comparable between allografts from the B7-1−/− recipient group and those in the wild-type recipient group. Relative to wild-type hosts, IL-13 expression trended lower in the B7-2−/− and B7-1/B7-2−/− recipient groups, although the differences were not statistically significant (Figure 3) ▶ . Except for IL-15 mRNA, which is constitutively expressed in heart tissue, 22 no or minimal gene expression of the tested cytokines and chemokines were observed in normal nontransplanted hearts. Relative to isografts, expression of all chemokines increased in allografts (data not shown). Most chemokines (lymphotactin, RANTES, eotaxin, MIP-β, MIP-1α, IFN-inducible protein-10, and monocyte chemoattractant protein-1) were minimally up-regulated in isografts compared to nontransplant normal hearts (data not shown). Isografts expressed negligible levels of cytokines except IL-15 and low-level IFN-γ.
Figure 3.
Representative panels and bar graphs showing the results of RPA. Ten micrograms of total RNA from each heart graft or normal heart was analyzed, using multiprobe RPA kit mCK-1 (containing DNA templates for IL-4, IL-5, IL-10, IL-13, IL-15, IL-9, IL-2, IL-6, IFN-γ, L32, and GAPDH). A: Representative gel images analyzed by mCK-1. B: Bar graph showing the results of densitometric analysis for IL-4 gene expression in the allografts (n = 3 for each group). IL-4 gene expression decreased significantly in the allografts in B7-2−/− and B7-1/B7-2−/− recipients compared with wild-type recipients. C: Bar graph showing the results of densitometric analysis for IFN-γ gene expression in the allografts (n = 3 for each group). IFN-γ gene expression was comparable in all allograft groups.
Distribution of Graft Infiltrating Macrophages, CD4(+) or CD8(+) T Cells, and B Cells
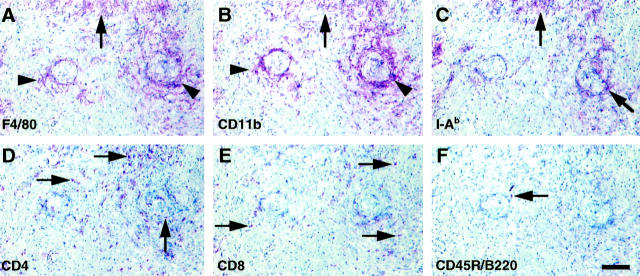
Sections in Figure 4 ▶ (A to F) show cross sections of coronary arteries (center) with perivascular mononuclear cellular infiltrates. Parenchymal mononuclear infiltrates are also shown (in the top fourth of each panel). Immunohistochemical staining for macrophages using two different monocyte/macrophage markers (F4/80 and CD11b) showed that the majority of graft-infiltrating cells are macrophages in all recipient genotypes (Figure 4) ▶ . Macrophages were primarily found in a periarteriolar distribution, whereas CD4(+) or CD8(+) cells were diffusely scattered. Some of the parenchymal lesions also included clusters of macrophages in close proximity to CD4(+) and occasional CD8(+) cells. Staining for recipient MHC class II molecules (I-Ab) co-localized with the macrophage-positive area, typically in a perivascular distribution. The allografts contained extremely few B cells and natural killer cells (not shown).
Figure 4.
Immunohistochemical staining for macrophages, recipient MHC class II molecules, CD4 (+) T cells, CD8 (+) T cells, or B cells at a low magnification; representative sections from a bm12 to B/6 wild-type allograft at 8 weeks after transplant. Scale bar = 100 μm. A: Anti-monocyte/macrophage marker F4/80 was used as a primary antibody. B: Anti-CD11b was used as another monocyte/macrophage marker. Both markers indicated similar distribution of positively stained areas including cluster of infiltrated macrophages in the parenchyma (arrows) and perivascular cuffing (arrowheads). C: Staining for recipient MHC class II molecule (I-Ab). Distribution of recipient MHC class II expressing cells was co-localized with areas of macrophage staining. Arrows indicate co-localizing I-Ab-positive staining area with macrophages. D: Staining for CD4 (+) T cells (arrows). E: Staining for CD8 (+) T cells (arrows). Immunostaining showed more scattered distribution of CD4 (+) or CD8 (+) T cells compared to macrophages. F: Staining for B cells (arrow). Anti-CD45R/B220 (pan-B cell marker) was used as primary antibody. B cells were barely detectable in the allografts.
Localization of B7-1 and B7-2 Molecules in the Allografts and Differential Expression on Graft Infiltrating Cells
B7-1 or B7-2 immunostaining co-localized with both the macrophage and I-Ab staining around vessels (Figure 5) ▶ . Allografts contained little or no CD40 or CD40 ligand (not shown). B7-1 and/or B7-2 molecules were not expressed on the graft-infiltrating cells in recipients lacking the corresponding gene, indicating that professional APC in the chronic stage grafts derive primarily from the recipient. Table 1 ▶ summarizes the results of immunohistochemistry.
Figure 5.
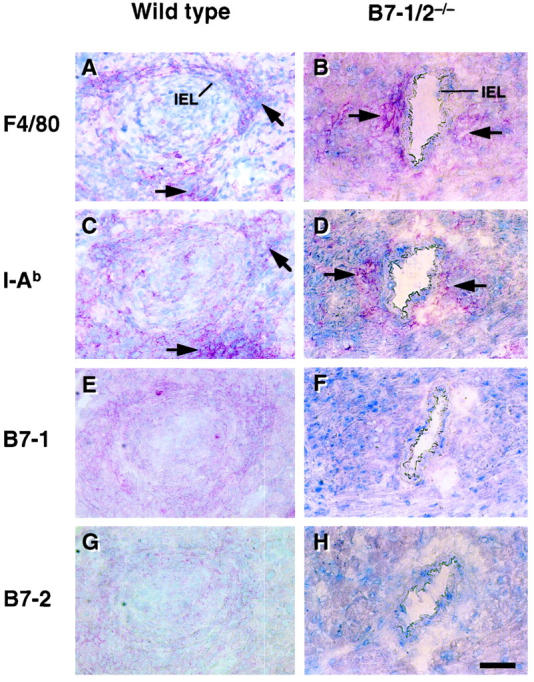
Immunohistochemical staining for macrophages, recipient MHC class II molecules, B7-1, or B7-2 molecules at a high magnification; representative sections from a bm12 to B/6 wild-type (A, C, E, and G) or a bm12 to B7-1/B7-2−/− (B, D, F, and H) allograft at 8 weeks after transplant. IEL, internal elastic lamina. Scale bar = 50 μm. A and B: F4/80 (macrophage marker) staining. C and D: I-Ab (MHC class II molecule marker for H-2b haplotype) staining. E and F: B7-1 staining. G and H: B7-2 staining. Adventitial and perivascular macrophage accumulation (A and B, arrows) was observed in most of the allografts, and was more severe in the diseased vessels. Positive staining for macrophages was co-localized with the distribution of recipient MHC class II molecules (C and D, arrows). Neither B7-1 nor B7-2 were stained in the allografts in B7-1/B7-2−/− recipients.
Table 1.
Summary of Immunohistochemistry of Grafts at 8 Weeks after Transplant
| Recipient genotype: bm12 → B/6 allografts | B/6 isografts | ||||
|---|---|---|---|---|---|
| Wild type | B7-1−/− | B7-2−/− | B7-1/2−/− | ||
| F4/80 | +++ | ++ | +++ | ++ | ± |
| CD4 | ++ | ++ | ++ | ++ | − |
| CD8 | + | + | + | + | − |
| CD45/B220 | + | ± | ± | ± | − |
| I-Ab | +++ | ++ | +++ | ++ | − |
| B7-1 | + | − | + | − | − |
| B7-2 | ± | ± | − | − | − |
| CD28 | + | + | + | + | − |
| CD40 | ± | ± | ± | − | − |
| CD40L | ± | − | ± | − | − |
Immunohistochemical staining of cell adhesion molecules was graded as: −, not present; ±, weak, focal; +, weak, diffuse; ++, moderate, focal; +++, moderate, diffuse; ++++, strong staining. Three to four samples were examined and the grades were averaged for each group. Details are described in Materials and Methods.
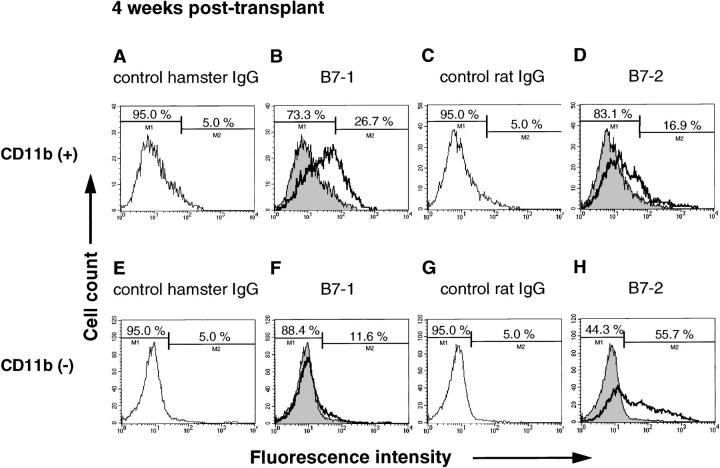
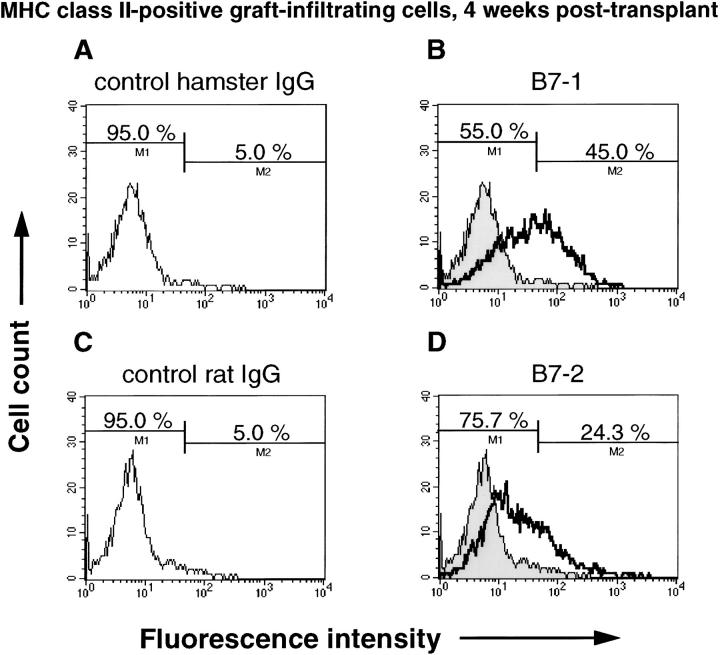
To further characterize B7 expression in chronic allografts, graft infiltrating cells in wild-type recipients were extracted from hearts and analyzed by flow cytometry. At 4 weeks after transplant, 26.7% of graft infiltrating CD11b(+) cells expressed B7-1, whereas only 16.9% of the CD11b(+) cells expressed B7-2 (Figure 6) ▶ . Similarly, at 8 weeks after transplant, 38.5% of CD11b(+) cells expressed B7-1, but only 14.8% of the CD11b(+) cells expressed B7-2 (data not shown). Conversely, >50% of the CD11b(−) cells expressed B7-2, whereas few CD11b(−) cells expressed B7-1 at 4 and 8 weeks (Figure 6) ▶ . Thus, macrophages in chronic allografts express significantly more B7-1 than B7-2, whereas nonmacrophages express more B7-2 than B7-1. Similar to these results, B7-1 was expressed by 45% of MHC class II(+) graft-infiltrating cells, whereas only 24.3% of MHC class II(+) cells stained positively for B7-2 (Figure 7) ▶ . Approximately 70% of the MHC class II(+) cells were also CD11b positive (data not shown) at 4 weeks.
Figure 6.
Flow cytometry of CD11b (+) (A–D) or CD11b (−) (E–H) graft infiltrating cells harvested from bm12 allografts in wild-type B/6 recipients at 4 weeks after transplant. Cells were double-stained using FITC-conjugated anti-CD11b antibody, and biotinylated isotype-matched hamster IgG, anti-B7-1 antibody, isotype-matched rat IgG or anti-B7-2 antibody, followed by PE-conjugated streptavidin. A: Histogram showing control staining for B7-1 staining gated on CD11b (+) population. The threshold was adjusted to 5% for background staining. B: Histogram showing B7-1 staining gated on CD11b (+) population. C: Histogram showing control staining for B7-2 staining gated on CD11b (+) population. The threshold was adjusted to 5% for background staining. D: Histogram showing B7-2 staining gated on CD11b (+) population. E: Histogram showing control staining for B7-1 staining gated on CD11b (−) population. The threshold was adjusted to 5% for background staining. F: Histogram showing B7-1 staining gated on CD11b (−) population. G: Histogram showing control staining for B7-2 staining gated on CD11b (−) population. The threshold was adjusted to 5% for background staining. H: Histogram showing B7-2 staining gated on CD11b (−) population.
Figure 7.
Flow cytometry of MHC class II (+) graft infiltrating cells harvested from bm12 allografts in wild-type B/6 recipients at 4 weeks after transplant. Cells were double-stained using FITC-conjugated anti-I-Ab antibody, and biotinylated isotype-matched hamster IgG, anti-B7-1 antibody, isotype-matched rat IgG or anti-B7-2 antibody, followed by PE-conjugated streptavidin, and then gated on I-Ab-positive (recipient MHC class II expressing) cells to analyze the population of each B7-1 or B7-2 expressing cells. A: Control staining for B7-1 using biotinylated isotype-matched hamster IgG. The threshold was adjusted to 5% for background staining. B: Staining using biotinylated anti-B7-1 antibody. Forty-five percent of the MHC class II (+) cells were also B7-1 positive. C: Control staining for B7-2 using biotinylated isotype-matched rat IgG. The threshold was adjusted to 5% for background staining. D: Staining using biotinylated anti-B7-2 antibody; 24.3% of the MHC class II (+) cells were also B7-2 positive.
Discussion
B7-1 and B7-2 co-stimulatory molecules are widely expressed on professional APC and provide the second signal for full activation of T lymphocytes. 5 Although B7-1 and B7-2 share some important characteristics, these molecules also show differences that indicate distinct roles for these two B7 molecules in chronic alloimmune responses. First, B7-1 and B7-2 molecules seem to differ in the kinetics of ligand-receptor engagement, in that B7-1 has a slower rate of association and dissociation with its counterreceptor CD28 than does B7-2. 23,24 Second, they have differentially regulated expression, depending on the cell populations and the nature of the stimuli. 25 In general, resting APC express B7-2 but not B7-1. 26 Both molecules are up-regulated on activation, with B7-2 levels rising and falling more quickly than B7-1 in vitro. However, dendritic cells may constitutively express both B7-1 and B7-2, and naive B cells may not constitutively express either molecule. 27,28 In the absence of immunosuppressive therapy, mouse cardiac allografts express B7-2 as early as 24 hours after transplant, followed by delayed expression of B7-1. 29 Thus, temporal differences between B7-1 and B7-2 expression also occur in vivo. These differences could conceivably result in distinct outcomes in host alloimmune responses. The effects of B7 co-stimulation on Th differentiation are not completely defined, 16,17,30,31 although both B7-1 and B7-2 co-stimulation seem to play important roles in driving naive Th0 cell to Th2 cells, 32 with B7-2 having a slightly stronger effect. 33 The differential effect on cytokine profiles may be attributed to temporal variations in expression or different kinetics of ligand-receptor engagement without requiring qualitative differences in their expression. 25,34
The present study used grafts at 8 weeks after transplant to evaluate GAD, immunohistology, cytokine expression, and B7 expression by graft-infiltrating cells in long-term allografts. We also analyzed B7 expression by graft-infiltrating cells at 4 weeks after transplant, earlier in the evolution of GAD. 13 The results establish that recipient B7-1 is more important than B7-2 in the development of GAD in MHC class II-disparate allografts in mice. At 4 or 8 weeks after transplant, B7-1 was significantly more expressed on MHC class II-positive APC than was B7-2, and B7-1−/− and B7-1/B7-2−/− recipients developed significantly less GAD than wild type. These effects of recipient B7 depletion most likely resulted from disruption of B7-1 and/or B7-2-CD28 engagement, because CD28 is the counterreceptor that transmits positive co-stimulatory signals from B7 and CTLA4 generally provides negative signals 35 by antagonizing B7-CD28 engagement or by inhibiting positive signals from T cell receptors. 36
We also observed a diminished IL-4 gene expression in the allografts lacking recipient B7-2 consistent with previous reports in other systems. 16,17 The Th1 cytokine IFN-γ was prominently expressed in all allografts, indicating B7-independent Th1 dominant alloimmune responses. Because IFN-γ levels were similar in allografts in wild-type recipients and in B7-1−/−, B7-2−/−, or B7-1/B7-2−/− recipients, neither B7-1 nor B7-2 seem strictly required in vivo for Th1 differentiation, consistent with our previous in vitro data. 32 This result also suggests that the Th1 type response driven by IFN-γ expression may overwhelm any expression of Th2 cytokines such as IL-4 and IL-13. As a result, small changes in IL-4 expression seem not to affect the development of GAD among the different recipient genotypes. Conversely, Th2 cytokines may have no role in ameliorating or augmenting GAD. 37 Although Th2 cytokines are frequently anti-inflammatory, and may be associated with allograft hyporesponsiveness or tolerance induction, 38-40 the precise role of Th2 cytokines in acute and chronic rejection still remains uncertain. 41-44 For example, long-term total allomismatched grafts in B7-1/B7-2 recipients are not associated with a Th2 immune deviation. 10 In addition, we found that augmentation of the Th2-type response by systemic administration of IL-10 exacerbated GAD in an MHC class II disparate mouse heart transplant model. 45 Thus, the balance of Th1 and Th2 cytokines is clearly not the only determinant for whether a graft experiences acute rejection or GAD.
We also investigated intragraft chemokine expression by RPA because chemokines are required for the effective recruitment of mononuclear inflammatory cells including T cells and monocytes/macrophages. The results demonstrated no differences between any of the allograft groups. Moreover, we did not find any significant decreases in mononuclear cell infiltrate in allografts placed in B7-1−/−, B7-2−/−, or B7-1/B7-2−/− recipients compared to wild type. This is consistent with our previous study, where we observed comparable mononuclear cell infiltrates in total allomismatched grafts placed in either B7-1/B7-2−/− or wild-type recipients at day 12 after transplant. 10 The findings indicate that the attenuated GAD in the B7-1−/− and the B7-1/B7-2−/− recipient groups does not result from differences in mononuclear cell recruitment into the allografts. Immunohistochemistry of graft infiltrating cells in each recipient genotype showed a similar pattern with a scattered distribution of CD4(+) or CD8(+) T cells and perivascular clustering of macrophages.
One potential explanation for GAD attenuation in the allografts of B7-1−/− and B7-1/B7-2−/− recipients is that B7-1 molecule expression on CD11b(+) and on MHC class II-expressing graft infiltrating cells at 4 or 8 weeks after transplant is critical for driving the alloresponse that will induce the vascular lesions. This observation complements recent studies suggesting a crucial role for early expression of B7-2 molecules in acute allograft rejection 29 and suggests distinctive roles for B7-1 and B7-2 molecules depending on the stage of rejection. In long-term allografts, recipient MHC class II and B7 molecules co-localized with a marked perivascular accumulation of macrophages around diseased vessels. These observations suggest the importance of chronic adventitial and perivascular inflammation in the pathogenesis of GAD, and a crucial role in this chronic inflammatory disorder for co-stimulatory signals from the interaction of B7-1 and CD28. Thus, B7-1-CD28 and B7-2-CD28 interactions may potentially provide distinct targets for immunosuppression in organ transplantation, depending on the stage of allograft rejection.
An important further observation concerns the relative importance of donor versus host co-stimulator expression. Because wild-type allografts implanted into recipients lacking B7-1 and B7-2 showed little B7 expression at 8 weeks, professional APC at a chronic stage are mostly of recipient origin. Although activated endothelial cells can express MHC class II, they do not express B7 molecules and it is unlikely that they function as APC in chronic-stage allografts. 46-48
In summary, B7-1 is the predominant B7 molecule expressed in chronic-stage allografts, and it is expressed on perivascular MHCII(+), CD11b(+) cells. Removal of B7-1-CD28 interaction resulted in significant reduction in GAD, whereas removal of B7-2-CD28 interaction did not. These results suggest that selective blockade of B7-1-CD28 pathway may provide a targeted therapeutic strategy for immunosuppression to ameliorate GAD.
Acknowledgments
We thank Ms. Eugenia Shvartz, Ms. Sarah Cole, Ms. Elissa Simon-Morrissey, Mr. Baolin Chang, and Ms. Karen E. Williams, Brigham and Women’s Hospital, for their skillful assistance.
Footnotes
Address reprint requests to Richard N Mitchell, M.D., Ph.D., Division of Immunology, Department of Pathology, Brigham and Women’s Hospital, Harvard Medical School, 221 Longwood Ave., Boston, MA 02115. E-mail: rmitchell@rics.bwh.harvard.edu.
Supported by National Institutes of Health Grants RO1 HL 43364 (to P. L. and R. N. M.), K11 AI01212 (to D. A. M.), and RO1 AI38310 (to A. H. S.). Yutaka Furukawa is a recipient of Research Fellowship Grant from Japan Heart Foundation.
References
- 1.Mason DW, Dallman MJ, Arthur RP, Morris PJ: Mechanisms of allograft rejection: the roles of cytotoxic T-cells and delayed-type hypersensitivity. Immunol Rev 1984, 77:167-184 [DOI] [PubMed] [Google Scholar]
- 2.Libby P, Tanaka H: The pathogenesis of coronary arteriosclerosis (“chronic rejection”) in transplanted hearts. Clin Transplant 1994, 8:313-318 [PubMed] [Google Scholar]
- 3.Bluestone JA: Costimulation and its role in organ transplantation. Clin Transplant 1996, 10:104-109 [PubMed] [Google Scholar]
- 4.Mondino A, Jenkins MK: Surface proteins involved in T cell costimulation. J Leukoc Biol 1994, 55:805-815 [DOI] [PubMed] [Google Scholar]
- 5.Greenfield EA, Nguyen KA, Kuchroo VK: CD28/B7 costimulation: a review. Crit Rev Immunol 1998, 18:389-418 [DOI] [PubMed] [Google Scholar]
- 6.Freeman GJ, Gray GS, Gimmi CD, Lombard DB, Zhou LJ, White M, Fingeroth JD, Gribben JG, Nadler LM: Structure, expression, and T cell costimulatory activity of the murine homologue of the human B lymphocyte activation antigen B7. J Exp Med 1991, 174:625-631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freeman GJ, Gribben JG, Boussiotis VA, Ng JW, Restivo VA, Jr, Lombard LA, Gray GS, Nadler LM: Cloning of B7-2: a CTLA-4 counter-receptor that costimulates human T cell proliferation [see comments]. Science 1993, 262:909-911 [DOI] [PubMed] [Google Scholar]
- 8.Azuma M, Ito D, Yagita H, Okumura K, Phillips JH, Lanier LL, Somoza C: B70 antigen is a second ligand for CTLA-4 and CD28. Nature 1993, 366:76-79 [DOI] [PubMed] [Google Scholar]
- 9.Yang Y, Wilson JM: CD40 ligand-dependent T cell activation: requirement of B7-CD28 signaling through CD40. Science 1996, 273:1862-1864 [DOI] [PubMed] [Google Scholar]
- 10.Mandelbrot DA, Furukawa Y, McAdam AJ, Alexander SI, Libby P, Mitchell RN, Sharpe AH: Expression of B7 molecules in recipient, not donor, mice determines the survival of cardiac allografts. J Immunol 1999, 163:3753-3758 [PubMed] [Google Scholar]
- 11.Glysing-Jensen T, Raisanen-Sokolowski A, Sayegh MH, Russell ME: Chronic blockade of CD28–B7-mediated T-cell costimulation by CTLA4Ig reduces intimal thickening in MHC class I and II incompatible mouse heart allografts. Transplantation 1997, 64:1641-1645 [DOI] [PubMed] [Google Scholar]
- 12.Russell PS, Chase CM, Winn HJ, Colvin RB: Coronary atherosclerosis in transplanted mouse hearts. I. Time course and immunogenetic and immunopathological considerations. Am J Pathol 1994, 144:260-274 [PMC free article] [PubMed] [Google Scholar]
- 13.Nagano H, Mitchell RN, Taylor MK, Hasegawa S, Tilney NL, Libby P: Interferon-gamma deficiency prevents coronary arteriosclerosis but not myocardial rejection in transplanted mouse hearts. J Clin Invest 1997, 100:550-557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng XX, Sayegh MH, Zheng XG, Li Y, Linsley PS, Peach R, Borriello F, Strom TB, Sharpe AH, Turka LA: The role of donor and recipient B7-1 (CD80) in allograft rejection. J Immunol 1997, 159:1169-1173 [PubMed] [Google Scholar]
- 15.Russell PS, Chase CM, Winn HJ, Colvin RB: Coronary atherosclerosis in transplanted mouse hearts. III. Effects of recipient treatment with a monoclonal antibody to interferon-gamma. Transplantation 1994, 57:1367-1371 [PubMed] [Google Scholar]
- 16.Kuchroo VK, Das MP, Brown JA, Ranger AM, Zamvil SS, Sobel RA, Weiner HL, Nabavi N, Glimcher LH: B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell 1995, 80:707–718 [DOI] [PubMed]
- 17.Freeman GJ, Boussiotis VA, Anumanthan A, Bernstein GM, Ke XY, Rennert PD, Gray GS, Gribben JG, Nadler LM: B7-1 and B7-2 do not deliver identical costimulatory signals, since B7-2 but not B7-1 preferentially costimulates the initial production of IL-4. Immunity 1995, 2:523-532 [DOI] [PubMed] [Google Scholar]
- 18.Freeman GJ, Borriello F, Hodes RJ, Reiser H, Hathcock KS, Laszlo G, McKnight AJ, Kim J, Du L, Lombard DB, Gray GS, Nadler LM, Sharpe AH: Uncovering of functional alternative CTLA-4 counter-receptor in B7-deficient mice. Science 1993, 262:907-909 [DOI] [PubMed] [Google Scholar]
- 19.Borriello F, Sethna MP, Boyd SD, Schweitzer AN, Tivol EA, Jacoby D, Strom TB, Simpson EM, Freeman GJ, Sharpe AH: B7-1 and B7-2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity 1997, 6:303-313 [DOI] [PubMed] [Google Scholar]
- 20.Corry RJ, Winn HJ, Russell PS: Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation 1973, 16:343-350 [DOI] [PubMed] [Google Scholar]
- 21.Stinn JL, Taylor MK, Becker G, Nagano H, Hasegawa S, Furukawa Y, Shimizu K, Libby P, Mitchell RN: Interferon-gamma-secreting T-cell populations in rejecting murine cardiac allografts: assessment by flow cytometry. Am J Pathol 1998, 153:1383-1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grabstein KH, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V, Beers C, Richardson J, Schoenborn MA, Ahdieh M, Johnson L, Alderson MR, Watson JD, Anderson DM, Giri JG: Cloning of a T cell growth factor that interacts with the β chain of the interleukin-2 receptor. Science 1994, 264:965-968 [DOI] [PubMed] [Google Scholar]
- 23.Linsley PS, Greene JL, Brady W, Bajorath J, Ledbetter JA, Peach R: Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. [Published erratum appears in Immunity 1995 Feb;2(2): following 203]. Immunity 1994, 1:793-801 [DOI] [PubMed] [Google Scholar]
- 24.van der Merwe PA, Bodian DL, Daenke S, Linsley P, Davis SJ: CD80 (B7-1) binds both CD28 and CTLA-4 with a low affinity and very fast kinetics. J Exp Med 1997, 185:393-403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawamura T, Furue M: Comparative analysis of B7-1 and B7-2 expression in Langerhans cells: differential regulation by T helper type 1 and T helper type 2 cytokines. Eur J Immunol 1995, 25:1913-1917 [DOI] [PubMed] [Google Scholar]
- 26.Freedman AS, Freeman GJ, Rhynhart K, Nadler LM: Selective induction of B7/BB-1 on interferon-gamma stimulated monocytes: a potential mechanism for amplification of T cell activation through the CD28 pathway. Cell Immunol 1991, 137:429-437 [DOI] [PubMed] [Google Scholar]
- 27.Boussiotis VA, Freeman GJ, Gribben JG, Daley J, Gray G, Nadler LM: Activated human B lymphocytes express three CTLA-4 counterreceptors that costimulate T-cell activation. Proc Natl Acad Sci USA 1993, 90:11059-11063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lenschow DJ, Zeng Y, Thistlethwaite JR, Montag A, Brady W, Gibson MG, Linsley PS, Bluestone JA: Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4lg. Science 1992, 257:789-792 [DOI] [PubMed] [Google Scholar]
- 29.Hancock WW, Sayegh MH, Zheng XG, Peach R, Linsley PS, Turka LA: Costimulatory function and expression of CD40 ligand, CD80, and CD86 in vascularized murine cardiac allograft rejection. Proc Natl Acad Sci USA 1996, 93:13967-13972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manickasingham SP, Anderton SM, Burkhart C, Wraith DC: Qualitative and quantitative effects of CD28/B7-mediated costimulation on naive T cells in vitro. J Immunol 1998, 161:3827-3835 [PubMed] [Google Scholar]
- 31.Greenwald RJ, Urban JF, Ekkens MJ, Chen S, Nguyen D, Fang H, Finkelman FD, Sharpe AH, Gause WC: B7-2 is required for the progression but not the initiation of the type 2 immune response to a gastrointestinal nematode parasite. J Immunol 1999, 162:4133-4139 [PubMed] [Google Scholar]
- 32.Schweitzer AN, Sharpe AH: Studies using antigen-presenting cells lacking expression of both B7-1 (CD80) and B7-2 (CD86) show distinct requirements for B7 molecules during priming versus restimulation of Th2 but not Th1 cytokine production. J Immunol 1998, 161:2762-2771 [PubMed] [Google Scholar]
- 33.Schweitzer AN, Borriello F, Wong RC, Abbas AK, Sharpe AH: Role of costimulators in T cell differentiation: studies using antigen-presenting cells lacking expression of CD80 or CD86. J Immunol 1997, 158:2713-2722 [PubMed] [Google Scholar]
- 34.Prabhu Das MR, Zamvil SS, Borriello F, Weiner HL, Sharpe AH, Kuchroo VK: Reciprocal expression of co-stimulatory molecules, B7-1 and B7-2, on murine T cells following activation. Eur J Immunol 1995, 25:207–211 [DOI] [PubMed]
- 35.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH: Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 1995, 3:541-547 [DOI] [PubMed] [Google Scholar]
- 36.Fallarino F, Fields PE, Gajewski TF: B7-1 engagement of cytotoxic T lymphocyte antigen 4 inhibits T cell activation in the absence of CD28. J Exp Med 1998, 188:205-210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mottram PL, Raisanen-Sokolowski A, Glysing-Jensen T, Stein-Oakley AN, Russell ME: Cardiac allografts from IL-4 knockout recipients: assessment of transplant arteriosclerosis and peripheral tolerance. J Immunol 1998, 161:602-609 [PubMed] [Google Scholar]
- 38.Mottram PL, Han WR, Purcell LJ, McKenzie IF, Hancock WW: Increased expression of IL-4 and IL-10 and decreased expression of IL-2 and interferon-gamma in long-surviving mouse heart allografts after brief CD4-monoclonal antibody therapy. Transplantation 1995, 59:559-565 [PubMed] [Google Scholar]
- 39.Punch JD, Tono T, Qin L, Bishop DK, Bromberg JS: Tolerance induction by anti-CD2 plus anti-CD3 monoclonal antibodies: evidence for an IL-4 requirement. J Immunol 1998, 161:1156-1162 [PubMed] [Google Scholar]
- 40.He XY, Chen J, Verma N, Plain K, Tran G, Hall BM: Treatment with interleukin-4 prolongs allogeneic neonatal heart graft survival by inducing T helper 2 responses. Transplantation 1998, 65:1145-1152 [DOI] [PubMed] [Google Scholar]
- 41.Lang T, Krams SM, Martinez OM: Production of IL-4 and IL-10 does not lead to immune quiescence in vascularized human organ grafts. Transplantation 1996, 62:776-780 [DOI] [PubMed] [Google Scholar]
- 42.Qian S, Li W, Li Y, Fu F, Lu L, Fung JJ, Thomson AW: Systemic administration of cellular interleukin-10 can exacerbate cardiac allograft rejection in mice. Transplantation 1996, 62:1709-1714 [DOI] [PubMed] [Google Scholar]
- 43.Onodera K, Hancock WW, Graser E, Lehmann M, Sayegh MH, Strom TB, Volk HD, Kupiec-Weglinski JW: Type 2 helper T cell-type cytokines and the development of “infectious” tolerance in rat cardiac allograft recipients. J Immunol 1997, 158:1572-1581 [PubMed] [Google Scholar]
- 44.Mueller R, Davies JD, Krahl T, Sarvetnick N: IL-4 expression by grafts from transgenic mice fails to prevent allograft rejection. J Immunol 1997, 159:1599-1603 [PubMed] [Google Scholar]
- 45.Furukawa Y, Becker G, Stinn JL, Shimizu K, Libby P, Mitchell RN: IL-10 augments allograft arterial disease: paradoxical effects of IL-10 in vivo. Am J Pathol 1999, 155:1929-1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Page C, Thompson C, Yacoub M, Rose M: Human endothelial stimulation of allogeneic T cells via a CTLA-4 independent pathway. Transplant Immunol 1994, 2:342-347 [DOI] [PubMed] [Google Scholar]
- 47.Rose ML: Endothelial cells as antigen-presenting cells: role in human transplant rejection. Cell Mol Life Sci 1998, 54:965-978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hasegawa S, Becker G, Nagano H, Libby P, Mitchell RN: Pattern of graft- and host-specific MHC class II expression in long-term murine cardiac allografts: origin of inflammatory and vascular wall cells. Am J Pathol 1998, 153:69-79 [DOI] [PMC free article] [PubMed] [Google Scholar]