Abstract
The emergence of cells with hepatocellular properties in the adult pancreas has been described in several experimental models. To determine whether adult pancreas contains cells that can give rise to therapeutically useful and biochemically normal hepatocytes, we transplanted suspensions of wild-type mouse pancreatic cells into syngeneic recipients deficient in fumarylacetoacetate hydrolase and manifesting tyrosinemia. Four of 34 (12%) mutant mice analyzed were fully rescued by donor-derived cells and had normal liver function. Ten additional mice (29%) showed histological evidence of donor-derived hepatocytes in the liver. Previous work has suggested that pancreatic liver precursors reside within or close to pancreatic ducts. We therefore performed additional transplantations using either primary cell suspensions enriched for ducts or cultured ducts. Forty-four mutant mice were transplanted with cells enriched for pancreatic duct cells, but only three of the 34 (9%) recipients analyzed displayed donor-derived hepatocytes. In addition, 28 of the fumarylacetoacetate hydrolase-deficient mice were transplanted with cultured pancreatic duct cells, but no donor-derived hepatocytes were observed. Our results demonstrate for the first time that adult mouse pancreas contains hepatocyte progenitor cells capable of significant therapeutic liver reconstitution. However, contrary to previous reports, we were unable to detect these cells within the duct compartment.
In embryonic development the liver and pancreas both originate from the same location in the ventral foregut. 1-5 The liver bud develops anteriorly toward the cardiac mesenchyme beginning at embryonic day 8.5 in the mouse. The pancreas has dorsal and ventral lobes with the dorsal bud growing in a posterior direction. The ventral lobe, however, develops more anteriorly and shares part of its ductal system with the liver. Because hepatocytes, bile ducts, pancreatic ducts, and exocrine and endocrine pancreatic cells are all endodermal in origin and because of the anatomical proximity of the developing liver and ventral pancreas, it is possible that all of these cell types are the offspring of a common stem cell. If this hypothesis is correct, adult animals might also have such hepatopancreatic stem cells with a broad differentiation potential for endodermal lineages, including hepatocytes and β-cells.
Several experimental models have indeed supported the hypothesis that multipotent stem cells persist in the adult pancreas and can give rise to a variety of differentiated offspring. In transgenic mice expressing interferon-γ under the transcriptional control of the insulin promoter, new β cells are generated de novo throughout the life of the animal from cells that reside within or close to the pancreatic ducts. 6,7 Another indication of the existence of pancreatic endodermal stem cells is the emergence of hepatocytes in the adult. The best known example is the appearance of hepatocytes in copper-depleted rats after re-feeding of copper. 8,9 In this system, weanling rats are fed a copper-free diet for 8 weeks that leads to complete acinar atrophy. When they are re-fed copper, cells with multiple hepatocellular characteristics emerge from the remaining pancreatic ducts within weeks. These cells have hepatocyte morphology and express a variety of hepatocyte markers, such as albumin. This work therefore suggested the presence of a pancreatic liver progenitor cell in or close to the pancreatic duct. 10 Copper depletion alone, without re-feeding, results in acinar atrophy and the proliferation of cells very similar to hepatic oval cells. 11 On transplantation into the liver, these pancreatic oval cells can differentiate and display multiple characteristics of hepatocytes. 11 More recently, specific cytokines have been identified as candidates to be involved in this process. Transgenic mice in which the keratinocyte growth factor gene is driven by insulin promoter consistently develop pancreatic hepatocytes. 12
The anatomical location of pancreatic stem cells has been determined to be in or near the ducts in both β-cell neogenesis and hepatocyte generation 6,10 Other work has also supported a direct role of pancreatic ducts in these processes. Cultured pancreatic duct cells were transplanted subcutaneously in the rat and then displayed hepatocyte markers such as albumin and α-fetoprotein. 13 Thus, pancreatic duct epithelium itself has been considered to represent a facultative stem cell by some investigators. 10,14
Although the earlier work described above had shown the existence of pancreas-derived cells expressing hepatocyte markers, it remains unknown whether these cells are fully functional and therefore therapeutically useful. Although pancreatic liver precursors seemed to be associated with the ducts, it remains unclear whether the ducts themselves contained the hepatocyte precursors or whether they are only anatomically close to the ducts (periductular). To address these questions, we tested adult murine pancreatic cells in the fumarylacetoacetate hydrolase (FAH) knockout liver repopulation model. 15-17
Here we report that pancreatic cells from adult mice contain hepatocyte progenitor cells that can significantly repopulate the livers in fumarylacetoacetate hydrolase-deficient (FAH−) mice 15,18,19 and reconstitute normal liver function. Furthermore, we provide evidence that these pancreatic liver progenitor cells are not the ducts themselves.
Materials and Methods
Strains of Mice and Animal Husbandry
As transplant recipients we used the FAHΔexon 5 strain mice previously described by this lab. 18 Transplant donors were transgenic ROSA-26 mice, a gift from P. Soriano (Fred Hutchinson Cancer Research Center, Seattle, WA). 20 All transplantation experiments were performed with congenic mice of the 129SvJ background. All FAH mutant animals were treated with NTBC-containing drinking water at a concentration of 7.5 mg/L (a gift from S. Lindstedt, Gøtheborg, Sweden). 21,22 This provides an approximate dose of 1 mg kg−1 body weight per day. For genotyping, polymerase chain reaction (PCR) was performed with a 3 primer PCR on 200-ng tail-cut DNA as previously described. Animal care and experiments were all in accordance with the Guidelines of the Department of Animal Care at Oregon Health Sciences University.
Isolation of Whole Pancreatic Cells
Whole pancreatic cells were isolated from adult (>3 months) male wild-type mice transgenic for Escherichia coli lacZ with a two step protease digestion. Briefly, the pancreas was dissected from sacrificed mice carefully avoiding injury of the liver. The harvested pancreas was immediately cut into small pieces and digested by collagenase D (Roche, Indianapolis, IN) (2.5 mg/ml, dissolved in Earle’s basic salt solution) for 25 minutes at 37°C with agitation by a magnetic stir bar. The cells were then pelleted by centrifugation, washed once with calcium-free phosphate-buffered saline (PBS), and then further digested in trypsin ethylenediaminetetraacetic acid (0.05% w/v) (Life Technologies, Inc., Rockville, MD) for 3 minutes. The proteases were neutralized by addition of 3 volumes of Dulbecco’s minimal essential medium (DMEM) with 10% bovine serum. Next, the digested cell mix was filtered through an 85-μm nylon mesh twice and the cells that came through the filter were collected. Cell number and viability were determined by Trypan blue exclusion staining under a hemocytometer.
Enrichment and Culture of Pancreatic Duct Cells
For pancreatic duct cell enrichment, a different protease digestion protocol was used. The chopped pancreas was first partially digested by collagenase D (Roche) (2.5 mg/ml, dissolved in Earle’s basic salt solution) for 20 minutes. The digestion was then filtered through a 85-μm nylon mesh. The cells retained on the mesh, enriched for ducts, were collected and then further digested under the same conditions for another 20 minutes.
Mouse pancreatic ducts were cultured by a published method 23 with some modifications. Briefly, the harvested pancreas was dissected from the animal and immediately chopped into small pieces. The tissue was then partially digested by collagenase D (0.7 mg/ml, Roche) for 20 minutes. The digestion mix was sieved through a nylon mesh attached to a glass funnel to remove the predominant acinar tissue. The nylon mesh with attached duct tissue was then cut from the funnel using sterile procedures and embedded in rat-tail collagen gel.
The preparation of rat collagen was modified from a previous report. 24 Briefly, the rat tail was harvested and sterilized in 70% ethanol. Then, the collagenous fibers of the tail were collected, weighed, and dissolved in 0.1% acetic acid (4.8 g/L) by stirring at 4°C for 3 to 5 days. The dissolved collagen solution was centrifuged at 10,000 × g at 4°C to remove the debris and sterilized by γ-irradiation (150 Gy). Before use, the acidic collagen stock solution was tested for its ability to form a gel at neutral pH. Eight times DMEM/F12 (Life Technologies, Inc.) medium was prepared, and two parts of 8× medium was combined with one part of serial NaOH stock solutions from 0.30 mol/L to 0.42 mol/L with 0.01 mol/L increments to make the serial neutralizing solutions. Then, one part of each of neutralizing solutions was mixed with 4.25 parts of acidic rat collagen stock solution. Each mixture was plated on each well of a 24-well culture plate. The plate was transferred to a tissue culture incubator with 5% CO2 and to equilibrate overnight. The desired result was neutral pH (based on the medium color) and a solid gel.
The cultures were fed with a DMEM/F12 (1:1) medium supplemented with 5% Nu-serum V (Collaborative Biomed, Bedford, MA), gentamicin (5 mg/L), and soybean trypsin inhibitor (100 mg/L; Sigma Chemical Co., St. Louis, MO), and a variety of factors including insulin (2.6 mg/L, Life Technologies, Inc.), murine epithelial growth factor (10 μg/L, Life Technologies, Inc.), dexamethasone (1 μmol/L, Sigma), and cholera toxin (100 μg/L, Life Technologies, Inc.). Half of the medium was exchanged every 3 days. After reaching confluency duct epithelial cells growing in the mesh were harvested by collagenase D (2.5 mg/ml, Roche) for 1 hour. The digestion was sieved by 85-μm size nylon mesh to collect single cells.
Pancreatic Cell Transplantation and Selection
The number and viability of harvested whole pancreatic cells, enriched duct cells, and cultured duct cells were determined by Trypan blue exclusion in a hemocytometer. The appropriate number of donor cells were resuspended in 100 μl of Dulbecco’s minimal essential media (Life Technologies, Inc.) with 10% fetal bovine serum and injected intrasplenically or directly into the portal vein of FAH mutant female recipient animals.
All FAH mutant transplant recipients were kept on NTBC until the time of transplantation. One day after transplantation they were switched to regular drinking water, not containing NTBC. The weight of experimental animals was measured weekly.
Biochemical Analysis
Samples from animals were obtained as follows. Animals were sacrificed by decapitation and blood collected by dabbing the wound onto parafilm (American National Can, Menasha, WI). For anticoagulation, the blood was immediately mixed with 10 μl of Na-heparin (Becton-Dickinson, Franklin Lakes, NJ) using a Pipetman. The red blood cells were removed by a brief centrifugation and the plasma was frozen at −80°C.
Twenty μl of plasma were mixed with 80 μl of a solution of 7% bovine serum albumin and assayed for aspartate serine aminotransferase, bilirubin, and creatinine levels with a Kodak Ektachem 700 chemistry analyzer (Eastman Kodak, Rochester, NY). Quantitative serum amino acid analyses were performed on a Beckman 6300 amino acid analyzer using published methodology. 25 Plasma succinylacetone levels in plasma were measured by a δ-aminolevulinate dehydratase inhibition assay as described. 26 FAH enzyme assays were performed at 30°C on a cytosolic fraction of homogenized liver as described previously. 27 Fumarylacetoacetate, the substrate for the assay, is not commercially available and was prepared enzymatically from homogentisic acid as described in the same reference. Protein concentrations were measured with a Bio-Rad kit (Bio-Rad, Richmond, CA). 28
Histology, Immune Histology, and Electron Microscopy
Liver tissues fixed in 10% phosphate-buffered formalin, pH 7.4, were dehydrated in 100% ethanol and embedded in paraffin wax at 58°C. Four-μm sections were rehydrated and stained with hematoxylin and eosin and with a polyclonal rabbit antibody to rat FAH (graciously provided by Robert Tanguay, University of Laval, Quebec, Canada) or glutamine synthetase. 29 The antibody was diluted in PBS, pH 7.4, and applied at concentrations of 1:300,000 at 37°C for 30 minutes. The glutamine synthetase antibody was used at a dilution of 1:10,000. Endogenous peroxidase activity was blocked with 3% H2O2 and methanol. Avidin and biotin pretreatment was used to prevent endogenous staining. The secondary antibody was biotinylated goat anti-rabbit IgG used at 1:250 dilution (BA-1000; Vector Laboratories, Burlingame, CA). Color development was performed with the AEC detection kit (catalogue no. 250–020; Ventana Medical Systems, Tucson, AZ).
β-galactosidase staining on the harvested liver tissues was performed. The liver tissues were fixed in 2% formaldehyde and 0.2% glutaraldehyde in PBS for 30 minutes. Then the tissues were washed with PBS for two times and moved to staining medium that contained 1 mg X-gal (Research Products International, Prospect, IL) in buffer of 5 mmol/L K ferricyanide, 5 mmol/L K ferrocyanide, 2 mmol/L MgCl2, 0.02% Nonidet P-40 (all Sigma), and 40 mmol/L Hepes (pH 8.0; Life Technologies). The staining incubation was at 37°C overnight. β-galactosidase histochemistry in frozen sections was performed as previously described. 16 The 8- to 10-μm-thick sections of OCT-embedded liver were fixed with 1.25% glutaraldehyde in ice-cold PBS for 10 minutes and stained overnight.
For detection of male cells, in situ hybridization was performed with a digoxigenin-labeled high-copy number Y chromosome-specific repeat DNA probe as previously described. 30
For electron microscopy, the cultured duct cells were harvested by collagenase D digestion. The cells were fixed in 3% glutaraldehyde buffered in 0.1 mol/L Na-cacodylate (pH 7.4). The cell samples were then postosmicated, embedded in araldite, sectioned on an AO-Reichert ultracut E microtome, and stained with uranyl acetate and lead citrate. Sections were examined in a Joel 100-CX microscope at magnifications ranging from ×800 to ×12,000.
Reverse Transcriptase (RT)-PCR and Northern Blots
Total cellular RNA of pancreatic duct cells were isolated from harvested pancreatic tissues and pancreatic duct cell cultures. 31 RT-PCR and Northern blots were performed according to standard protocols. 31 For the detection of the pancreatic duct markers carbonic anhydrase type II (CAII) and cystic fibrosis transmembrane regulator (CFTR), specific primers for both cDNA fragments were generated based on the published CAII (GenBank accession, K00811) and CFTR (GenBank accession, M69289) sequences. The primer pairs for CAII were: forward 5′-GGAGACCGGCAGTCCCCTGT-3′ and reverse 5′-AGAGAGGCGGTCACACTTGT-3′. The primer pairs for CFTR were: forward 5′ACCCTTGTGGATGGGGGTTATGTGC-3′ and reverse 5′-CATGGGTTCTGGGAATGGACTC-3′. RT was performed by using 2 μg of total RNA samples with random hexamers and M-MLV reverse transcriptase (Life Technologies, Inc.). The PCR conditions were: 94°C for 5 minutes; 95°C for 1 minute, 60°C for 1 minute, and 72°C for 2 minutes, for 35 cycles; 72°C for 10 minutes for ending. After RT-PCR, the DNA fragments were resolved on a 0.8% agarose gel.
To further estimate the relative quantities of CFRT mRNA in different cells we used a 4-primer RT-PCR with the housekeeping gene APRT (adenine phosphoribosyltransferase) as an internal control. The primer pairs for APRT were: forward 5′-AGCGTGCTGATACCTACCTC-3′ and reverse 5′-AAGCAGTTCCTAGTGCTGCT-3′. The PCR conditions were: four primers added in a single reaction, including 40 ng of each CFTR primers and 200 ng of APRT primers in 25 μl reaction; 94°C for 5 minutes for starting; 95°C for 1 minute, 60°C for 1 minute, and 72°C for 2 minutes, for 28 cycles; 72°C for 10 minutes for ending. After RT-PCR, the DNA fragments were resolved on a 1.5% agarose gel.
Northern blots were probed with both RT-PCR generated cDNA fragments as probes. Fifteen μg of total RNA was separated on the formaldehyde denature gel and transferred onto Hybond-N nylon membrane. The hybridization was performed in 50% formamide hybridization solution the α-32P-dCTP labeled probes (5 × 10 6 cpm/ml) at 42°C for 18 hours. The radioactive signals were shown by the exposure on the X-ray film.
Results
Transplantation of Unfractionated Pancreatic Cell Suspensions
To test the hypothesis that pancreas contains a population of liver progenitor cells, we harvested cells from whole pancreas of adult (>3 months) male, FAH wild-type ROSA-26 mice transgenic for an ubiquitously expressed E. coli lacZ gene. 20 A single cell suspension was transplanted into 10-week-old female FAH− mutant congenic recipients. Three to five × 10 5 crude pancreatic cells were injected into the spleens of the FAH− recipients and NTBC treatment was discontinued immediately after transplantation to permit selection of FAH+ hepatocytes. 15 Our previous work with hepatocyte transplantation has shown that liver repopulation is only partial at 4 weeks of selection and is near-complete after 8 weeks. 15 Even nontransplanted FAH mutants typically survive 6 to 8 weeks off NTBC. 22 Therefore, transplanted recipients were divided into two groups for repopulation analysis: one group was harvested at 4 weeks after transplantation, whereas the other group was harvested after 8 weeks. In total, 68 FAH mutants were transplanted in 12 independent experiments. During the selection period, weights of the transplant recipients were followed weekly and all recipients lost weight during the first 4 weeks of transplantation. In the early harvesting group with a total of 28 recipients, nodules of FAH+ hepatocytes were found in 10 (29%) of the transplanted FAH− recipients. In these animals, only a single 4- to 5-μm liver section representing <0.1% of the total liver was analyzed. The FAH+ nodules were small with 6 to 20 cells visible in cross-section, indicating that three-dimensionally each clone contained ∼10 to 50 cells (Figure 1A) ▶ . In the 8-week-time (late) observation group of 40 recipients, most of transplanted FAH− recipients died with tyrosinemic symptoms for liver failure before 6 weeks, ie, before the scheduled harvesting time. However, six of 40 transplanted mice regained the originally lost weight and completely recovered at 6 to 8 weeks after transplantation. Of these harvested at 8 weeks, four recipients (generated in three independent transplantation experiments) had from 50 to 90% FAH+ hepatocyte repopulation as shown by the immunohistochemistry (Figure 1B) ▶ . The other two recipients had developed hepatocarcinoma thus making evaluation of liver function impossible. The carcinomas originated in recipient tissue, not transplanted donor cells, as has been reported previously reported in the FAH knockout model. 32,33 To confirm that the repopulating cells were indeed donor-derived, we performed β-galactosidase staining (Figure 2C) ▶ and Y chromosome in situ hybridization (Figure 2A) ▶ . Because all of the transplantations were performed by transplanting of Rosa-26 male pancreatic cells into FAH− female recipients, the donor cells could readily be distinguished from host cells (Figure 2) ▶ .
Figure 1.
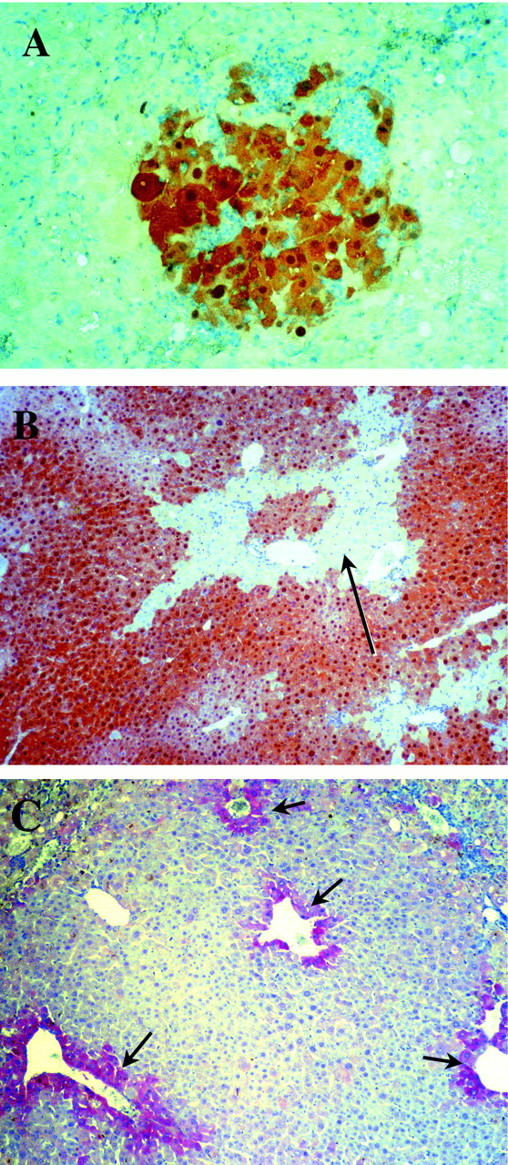
FAH mutant animals repopulated with wild-type pancreatic cells. A and B: FAH immunohistochemistry in the liver of FAH mutant mice transplanted with pancreatic cells. A: Small nodule of pancreas derived 4 weeks after transplantation. Original magnification, ×200. B: Almost complete repopulation by pancreas-derived hepatocytes after 8 weeks after transplantation. Only small pockets of mutant tissue (black arrow) remained. Original magnification, ×100. C: Immunohistochemistry with an antibody to glutamine synthetase in a liver extensively repopulated by pancreatic donor cells. Glutamine synthetase was expressed only in zone 3 hepatocytes in the repopulated areas (black arrow), similar to that of normal wild-type control. Original magnification, ×100.
Figure 2.
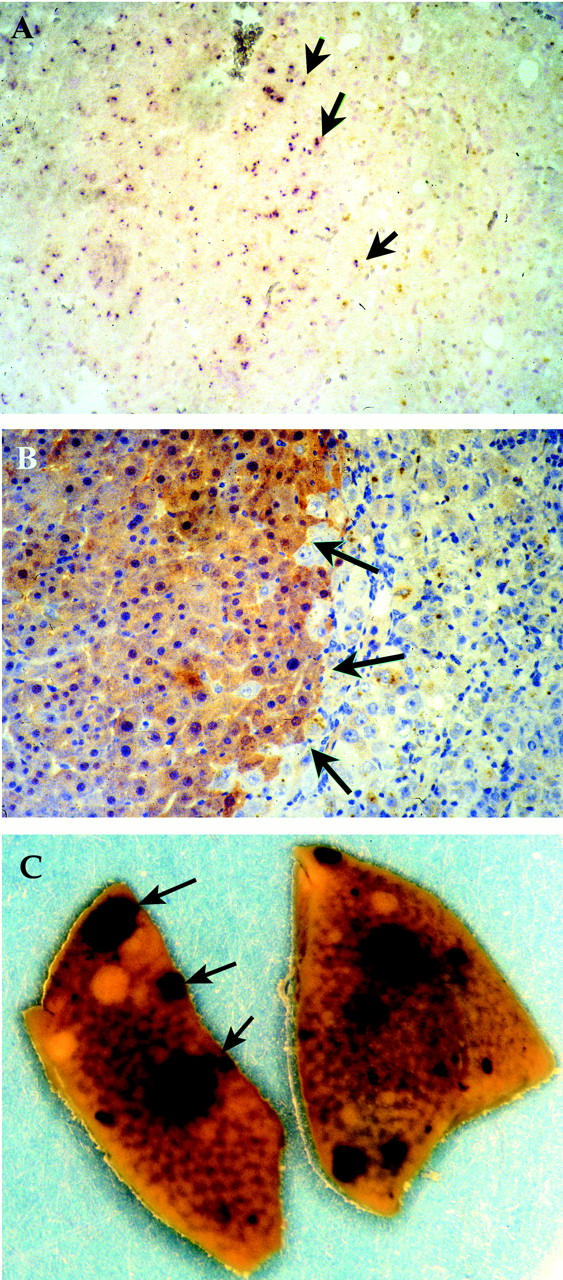
Y chromosome FISH and β-galactosidase staining of repopulated liver. All sections were from a female FAH− recipient repopulated with male donor pancreatic cells 8 weeks after transplantation. In situ hybridization was performed with a digoxigenin-labeled high-copy number Y chromosome-specific repeat DNA probe. A: Y chromosome FISH of a repopulation nodule. The nuclei of donor-derived hepatocytes are Y chromosome-positive (arrows). B: FAH immunohistochemistry of the same nodule. Original magnification, ×200. C: Whole-mount β-galactosidase staining of FAH mutant liver 4 weeks after transplantation with pancreatic cells. Donor-derived hepatocyte nodules stained blue (arrows).
FAH enzyme activity was measured in the four repopulated livers without hepatocarcinoma and ranged from 30 to 90% of wild-type control levels, consistent with the immunohistochemistry results. To determine whether the liver function of the four long-term survivors was normal, several parameters of hepatocellular function were measured in blood, including aspartate serine aminotransferase, bilirubin, and the levels of amino acids (Table 1) ▶ . These values were compared to wild-type controls and untransplanted FAH mutant mice off NTBC (Table 1) ▶ . Only the alanine aminotransferase (ALT) and tyrosine levels were mildly elevated in the FAH mutant mice repopulated with pancreas-derived cells compared to wild type. This result is similar to those obtained with transplantation of hepatocytes, in which repopulation of <80% also results in mildly abnormal ALT and tyrosine. 16 Importantly, the plasma level of succinylacetone, the hallmark metabolite for tyrosinemia was completely normalized in pancreatic cell-transplanted mutants. Furthermore, the normal plasma levels of all amino acids represents a global measure of many hepatic enzymes including the urea cycle (glutamine, citrulline, arginine) and glucose metabolism (alanine). The expression pattern of glutamine synthetase was also analyzed by immunohistochemistry in the repopulated livers, and found to be limited to zone 3 hepatocytes adjacent to hepatic veins as in wild-type controls (Figure 1C) ▶ . This indicates that the normal zonular organization of the liver lobule was re-established with the repopulation. 29
Table 1.
Biochemical Measures of Liver Function
| Liver function parameter | Units | Plasma levels ± SD | P values | |||
|---|---|---|---|---|---|---|
| Wild type | PCT mutant* | Untreated mutant | PCT versus wild type | PCT versus mutant | ||
| FAH activity | μmoles/min · g | 42.8 ± 1.3 (6) | 29 ± 13 (6) | 0.26 ± 0.22 (6) | 0.052 ns | 0.0012 |
| Succinylacetone | μg/L | 3.8 ± 1.9 (6) | 4.71 ± 1.6 (6) | 186 ± 11 (4) | 0.73 ns | <0.0001 |
| ALT | U/L | 50 ± 33 (21) | 176 ± 28 (6) | 466 ± 69 (9) | 0.0001 | 0.006 |
| Total bilirubin | mg/dL | 0 (21) | 0.27 ± 0.2 (6) | 7.1 ± 2.9 (6) | ns | <0.0001 |
| Tyrosine | μmol/L | 51 ± 15 (9) | 126 ± 39 (6) | 1000 ± 140 (6) | 0.0006 | <0.0001 |
| Phenylalanine | μmol/L | 68 ± 5 (9) | 54 ± 9 (6) | 180 ± 76 (6) | 0.08 ns | 0.014 |
| Methionine | μmol/L | 44 ± 5 (9) | 45 ± 8 (6) | 230 ± 100 (6) | 0.9 ns | 0.0016 |
| Glutamine | μmol/L | 489 ± 105 (9) | 534 ± 178 (6) | 5800 ± 1500 (6) | 0.66 ns | 0.0001 |
| Glycine | μmol/L | 260 ± 49 (9) | 266 ± 69 (6) | 930 ± 400 (6) | 0.97 ns | 0.004 |
| Alanine | μmol/L | 340 ± 140 (9) | 269 ± 91 (6) | 1800 ± 750 (6) | 0.88 ns | 0.03 |
| Valine | μmol/L | 176 ± 7 (9) | 140 ± 22 (6) | 336 ± 43 (6) | 0.06 ns | 0.0023 |
Measurements are given for littermate wild-type controls, FAH mutants rescued with pancreatic cell transplantation (PCT), and untreated FAH mutants off NTBC for 5 weeks. The number of mice analyzed is given in brackets. P values were calculated using the two-tailed t-test; ns, nonsignificant (>0.05).
*Including four mice from transplantation group with unfractionated pancreatic cell suspension and two mice from transplantation group with cell suspensions enriched for pancreatic ducts.
Together these results show conclusively that normal adult mouse pancreas contains transplantable cells that can differentiate into fully functional hepatocytes and cause near-complete liver repopulation in FAH mutant mice.
Serial Transplantation of Hepatocytes Derived from Pancreatic Cells
To address whether the pancreatic cell-derived FAH+ hepatocytes retain their regenerative capacity, we serially retransplanted 1 × 10 5 cells from two repopulated livers into additional FAH− recipients. With the first of these donors, two additional rounds of serial transplantation were successful, resulting in phenotypic correction of two generations of FAH mutants. FAH− immunohistochemistry and β-galactosidase staining were used to measure the degree of repopulation, which exceeded 50% in all cases (data not shown). The number of cell divisions required to achieve 50% repopulation is estimated to be ∼10. 16 Therefore at least 20 cell divisions are needed to obtain the sequential repopulation observed. The second donor was used for only one additional round of serial transplantation, again resulting in a high percentage (>50%) of repopulation in the secondary recipients. These results show that pancreas-derived hepatocytes have a regenerative capacity similar to liver-derived hepatocytes.
Transplantation of Cell Suspensions Enriched for Pancreatic Ducts
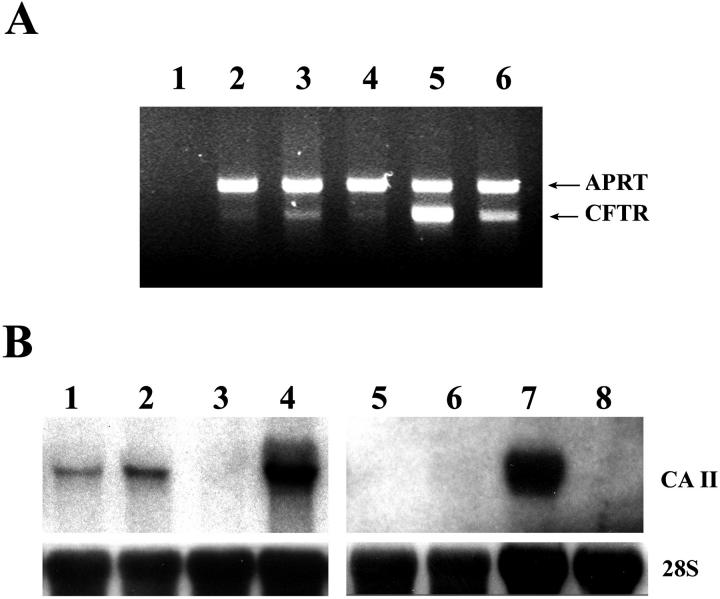
Previous reports suggested that both pancreatic liver stem cells and the cells responsible for β-cell neogenesis reside in the pancreatic duct system. 10,14 We therefore hypothesized that pancreatic cell suspensions enriched for duct cells would be superior for liver repopulation compared to cell suspensions containing all pancreatic cell types. We designed two methods to enrich for transplantation of duct cells: First, we concentrated ducts from freshly isolated pancreas by performing partial collagenase digestion and then filtering through a nylon mesh to capture the undigested duct fragments for further digestions into a single cell suspension. Second, we established high-density primary cultures of duct cells (see below). For the first approach, two steps of collagenase digestions were used to enrich the pancreatic duct cells. The chopped whole pancreas was first digested mildly by collagenase D to remove the acinar cells. The undigested part contained mainly of the duct tissues and was collected by being retained on the filter after being passed through a nylon mesh. Figure 3A ▶ shows the morphology of the intact ducts collected by this method before the second digestion step. The enrichment for duct cells was verified by performing Northern blots and RT-PCR for two specific markers, the CFTR and CAII (Figure 4) ▶ .
Figure 3.
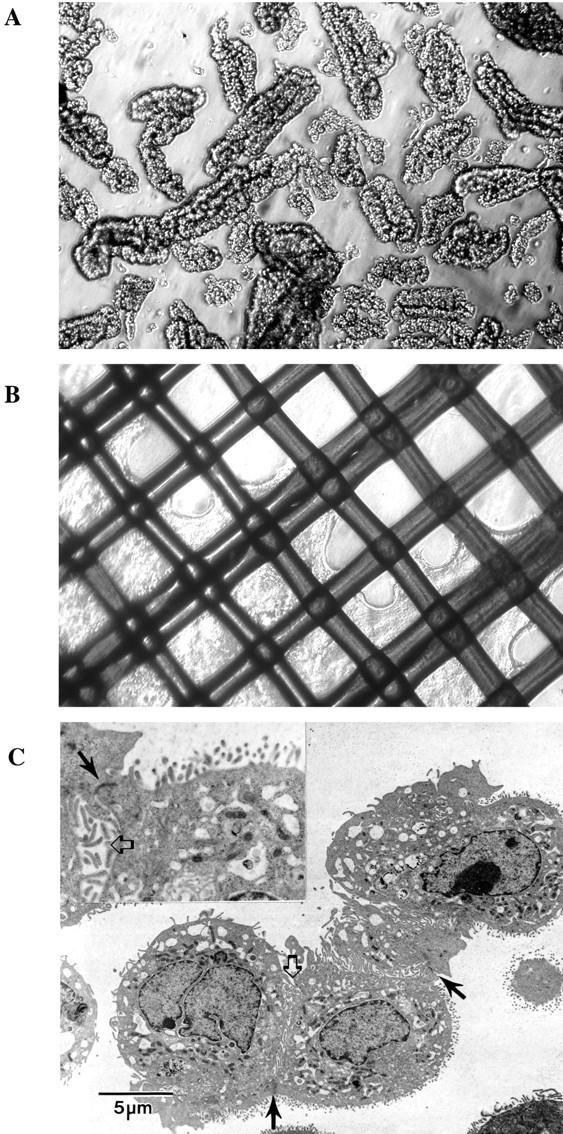
Enriched pancreatic ducts and pancreatic duct epithelium in culture. A: Enriched pancreatic ducts after partial digestion with collagenase D and filtration through 85-μm nylon mesh. Phase contrast, original magnification, ×100. B: Primary culture of duct cells embedded in rat-tail collagen gel with a nylon mesh. Expanding duct epithelium can be seen in the lattice. Original magnification, ×100. C: Electron microscopy of the cultured pancreatic duct cells. Their epithelial nature was apparent by the prominent microvillus processes along portions of the plasma membrane (thick white arrows) and formed tight junctions (solid black arrows) between the cohered cells. No secretary granules are seen to suggest acinar cell derivation.
Figure 4.
Expression of CAII and CFTR in enriched pancreatic ducts and pancreatic duct epithelium primary culture. A: RT-PCR to estimate expression of CFTR mRNA compared with the housekeeping gene APRT. The RT-PCR product of APRT (642-bp band) amplified equally in all samples. The RT-PCR product of CFTR (488-bp band) was strongest in the sample of pancreatic duct epithelium culture (lane 5). Enriched pancreatic ducts (lane 3) had higher expression than whole pancreas (lane 2) or enriched acinar cells (lane 4). Lane 1: No RT control; lane 6: positive control, mouse kidney. B: Northern blot for comparison of CAII expression levels. Left: lane 1, whole pancreas; lane 2, enriched ducts; lane 3, enriched acinar cells; lane 4, mouse kidney, a positive control for the expression of CAII. Right: lane 5, whole pancreas; lane 6, enriched acinar cells; lane 7, cultured pancreatic duct epithelium; lane 8, enriched ducts placed in culture without collagen matrix.
For transplantation 5 × 10 5 viable cells were injected into the spleens of FAH mutant recipients. There were 44 transplanted mice. Thirty mice were harvested early and the other 14 mice remained for long-time observation. Finally, only two mice survived with recovered liver function after 8 weeks of transplantation. As indicated in Table 2 ▶ , only three of 34 (9%) of the animals analyzed had any evidence for hepatocyte repopulation. Thus, contrary to our expectation, enrichment for duct cells did not enhance liver repopulation.
Table 2.
Summary Results of Pancreatic Cell Transplantation into FAH Mutant Mice
| Method of pancreatic cell preparation | Transplanted mice | Mice analyzed (early/late) | Mice with FAH+ nodules* | Mice with significant liver repopulation† | Total number of mice with repopulation‡ |
|---|---|---|---|---|---|
| Filtered single cells | 68 | 34 (28/6) | 10/34 (29%) | 4/34 (12%) | 14/34 (41%) |
| Enriched duct cells | 44 | 34 (30/4) | 1/34 (3%) | 2/34 (6%) | 3/34 (9%) |
| Cultured ducts | 28 | 14 (14/0) | 0/14 | 0 | 0/14 |
*Fraction of mice analyzed early that had FAH+ nodules.
†Fraction of mice analyzed late that had significant FAH+ repopulation.
‡Fraction of all mice that could be analyzed. Animals that died and two animals with cancer were excluded.
Transplantation of Cultured Pancreatic Duct Cells
We next established an improved method for culturing primary pancreatic duct cells. In a modification of a previously published technique, 23,34 ducts collected on a nylon mesh were not removed before embedding in collagen, but rather were placed into the gel together with the mesh. This led to a rapid outgrowth of duct epithelium at high density and enhanced the yields of duct epithelial cell fivefold to 10-fold compared with the previous method. The duct cells in the collagen gel became confluent on the mesh lattice 2 to 3 weeks after plating, yielding ∼10 million pure pancreatic duct epithelial cells from a single pancreas. The phenotypic identity of the cultured cells was verified by two methods. First, the cultured cells were shown to have typical duct epithelium morphology by phase microscopy (Figure 3B) ▶ and electron microscopy (Figure 3C) ▶ . Second, the expression of the marker genes CFTR and CAII was demonstrated by RT-PCR and Northern blot (Figure 4, A and B) ▶ . Both markers were highly expressed. For transplantation, confluent duct epithelium was harvested by incubation with collagenase D followed by trypsin/ethylenediaminetetraacetic acid and 5 × 10 5 viable cells were injected into the spleen of FAH mutant recipients. A total of 28 FAH mutant mice were transplanted. None of the animals survived the complete selection period of 8 weeks, and none of the 14 animals harvested at 4 weeks for histological analysis had any evidence of donor cell engraftment in the liver. Thus, cultured primary pancreatic duct epithelium was not able to undergo hepatocyte differentiation in our in vivo repopulation assay.
Discussion
In embryonic development, a common endodermally derived precursor in the ventral foregut is thought to give rise to both exocrine and endocrine pancreatic cell lineages 5,35 and several lines of evidence strongly support the existence of pluripotent stem cells in the adult pancreas. β-cell neogenesis can occur in adult animals 6 and cells with hepatocyte properties can develop under a variety of experimental conditions. 10,12 Both islet cells and hepatocytes generated de novo originate from within or near pancreatic ducts. 6,10 This shared anatomical location and the common embryological roots of liver and pancreas suggest the hypothesis that the same cell may be responsible for β-cell neogenesis and pancreatic hepatocyte formation. 35 However, neither β-cell precursors nor the progenitor of pancreatic hepatocytes have been isolated to answer this question, because to date assays suitable for the isolation of pancreatic stem cells have not been developed.
In the past, it was not possible to determine whether pancreatic hepatocytes simply represented transdifferentiation and metaplasia under pathological conditions or if they were true hepatocytes, derived from an undifferentiated progenitor cell (stem cell). In the copper-depleted rats and keratinocyte growth factor transgenic mice, pancreatic hepatocytes display many characteristics of immature liver cells, such as expression of α-fetoprotein, also seen in hepatocarcinoma. 11,12 The experiments reported here demonstrate unambiguously that the pancreas of the normal adult mouse contains undifferentiated progenitors of fully functional hepatocytes. These transplantable cells can phenotypically ameliorate the tyrosinemic liver disease of FAH mutant mice and their progeny are indistinguishable from liver resident hepatocytes. As in previous work resulting in partially repopulated tyrosinemic mice, liver functions that reflect cell autonomous properties of FAH mutant hepatocytes (ie, hepatocarcinoma, release of transaminases, release of tyrosine) were still slightly abnormal here also. 16,32,33 This observation does not indicate incomplete differentiation of pancreas-derived hepatocytes, but rather reflects the cell autonomous nature of liver disease in FAH deficiency. 33
The data support the concept of a pancreatic stem cell able to differentiate into the hepatic lineage. The same cell may also be able differentiate into different pancreatic lineages and thus could be multipotent. The frequency and exact phenotypic identity of this stem cell were not ascertained in the experiments reported here. However, we did seek to determine whether pancreatic ducts harbor the stem cells as previously suggested. Our results indicated that differentiated ducts themselves were not directly responsible for liver repopulation, but did not exclude the possibility that the repopulating cell was anatomically close to the ducts. Mild collagenase digestion may separate the pancreatic hepatocyte progenitors from the ducts, with which it may be anatomically associated in situ. Such transitional cells located between the ducts and the parenchyma may represent the stem cell fraction. Others have recently reported β-cell neogenesis from cultured pancreatic duct cells. 14,36 Despite this claim, however, the authors did not demonstrate that the β cells originated from ducts themselves rather than periductular cells. Thus, it is still possible that the pancreatic hepatocyte progenitors described here and β-cell progenitors reported by others might be identical. Alternatively, two distinct progenitor populations may exist.
It is unlikely that pancreatic liver stem cells would find a therapeutic application for the treatment of liver diseases. The pancreas resides in an anatomically inaccessible location, making it a poor source of cells for liver repopulation. Furthermore, the efficiency of liver repopulation by pancreatic cells was inferior to what can be achieved with liver-derived hepatocytes. Despite transplantation of a large number of donor cells, only a small fraction of animals had significant degrees of liver replacement. The significance of the work reported here lies in the description of an in vivo transplantation assay for pancreatic stem cells. Our data clearly indicate that the liver repopulating cells reside in a single cell suspension. Therefore, fractionation of the cell suspension into subpopulations should be possible by standard cell sorting methods such as fluorescence-activated cell sorting for cell surface markers. Using such methodology the purification and isolation of pancreatic liver precursors should be possible. Because of the common embryological origin of liver and pancreas, it is possible that this liver stem cell may also have the potential to give rise to other endodermal cell types. Therefore liver repopulation by pancreatic donor cells may serve as a surrogate assay for pluripotent stem cells and could potentially be used to purify stem cells capable of β-cell neogenesis.
The findings reported here are also relevant to the issue of liver regeneration. It has been controversial for some time whether stem cells are sometimes involved in reconstitution of the adult liver. 37,38 Our own work has shown that hepatocytes themselves are highly efficient in liver repopulation. 17 The results obtained here clearly indicate that therapeutic liver repopulation is also possible with undifferentiated progenitors. Whether transplantation of stem cells has practical advantages compared to transplantation of hepatocytes currently remains unknown, but it is conceivable that they could be useful for the treatment of liver disease that is not limited to hepatocytes.
Acknowledgments
We thank Dr. M. Gibson from the biochemical genetics laboratory at Oregon Health Sciences University for the analysis of plasma amino acids; Dr. C. N. Ou (Baylor College of Medicine, Houston, TX) for the automated analysis of liver functions; A. Major for technical assistance with the immunohistochemistry; and J. Barrish for help with the electron microscopy.
Footnotes
Address reprint requests to Markus Grompe, M.D., Dept. of Molecular and Medical Genetics, Oregon Health Sciences University, 3181 SW Sam Jackson Pk. Rd., L103, Portland, OR 97201. E-mail: grompem@ohsu.edu.
Supported by National Institutes of Health NIDDK grant D-51592 (to M. G.) and Mel Howard Research Fellowship from American Liver Foundation (to X. W.).
References
- 1.Zaret KS: Molecular genetics of early liver development. Annu Rev Physiol 1996, 58:231-251 [DOI] [PubMed] [Google Scholar]
- 2.Zaret KS: Liver specification and early morphogenesis. Mech Dev 2000, 92:83-88 [DOI] [PubMed] [Google Scholar]
- 3.Gualdi R, Bossard P, Zheng M, Hamada Y, Coleman JR, Zaret KS: Hepatic specification of the gut endoderm in vitro: cell signaling and transcriptional control. Genes Dev 1996, 10:1670-1682 [DOI] [PubMed] [Google Scholar]
- 4.Spooner BS, Walther BT, Rutter WJ: The development of the dorsal and ventral mammalian pancreas in vivo and in vitro. J Cell Biol 1970, 47:235-246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rutter WJ: The development of the endocrine and exocrine pancreas. Monogr Pathol 1980, 21:30-38 [PubMed] [Google Scholar]
- 6.Gu D, Sarvetnick N: Epithelial cell proliferation and islet neogenesis in IFN-g transgenic mice. Development 1993, 118:33-46 [DOI] [PubMed] [Google Scholar]
- 7.Gu D, Arnush M, Sawyer SP, Sarvetnick N: Transgenic mice expressing IFN-gamma in pancreatic beta-cells are resistant to streptozotocin-induced diabetes. Am J Physiol 1995, 269:E1089-E1094 [DOI] [PubMed] [Google Scholar]
- 8.Rao MS, Subbarao V, Reddy JK: Induction of hepatocytes in the pancreas of copper-depleted rats following copper repletion. Cell Differ 1986, 18:109-117 [DOI] [PubMed] [Google Scholar]
- 9.Rao MS, Dwivedi RS, Subbarao V, Usman MI, Scarpelli DG, Nemali MR, Yeldandi A, Thangada S, Kumar S, Reddy JK: Almost total conversion of pancreas to liver in the adult rat: a reliable model to study transdifferentiation. Biochem Biophys Res Commun 1988, 156:131-136 [DOI] [PubMed] [Google Scholar]
- 10.Rao MS, Yeldandi AV, Reddy JK: Stem cell potential of ductular and periductular cells in the adult rat pancreas. Cell Differ Dev 1990, 29:155-163 [DOI] [PubMed] [Google Scholar]
- 11.Dabeva MD, Hwang SG, Vasa SR, Hurston E, Novikoff PM, Hixson DC, Gupta S, Shafritz DA: Differentiation of pancreatic epithelial progenitor cells into hepatocytes following transplantation into rat liver. Proc Natl Acad Sci USA 1997, 94:7356-7361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krakowski ML, Kritzik MR, Jones EM, Krahl T, Lee J, Arnush M, Gu D, Sarvetnick N: Pancreatic expression of keratinocyte growth factor leads to differentiation of islet hepatocytes and proliferation of duct cells. Am J Pathol 1999, 154:683-691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen JR, Tsao MS, Duguid WP: Hepatocytic differentiation of cultured rat pancreatic ductal epithelial cells after in vivo implantation. Am J Pathol 1995, 147:707-717 [PMC free article] [PubMed] [Google Scholar]
- 14.Ramiya VK, Maraist M, Arfors KE, Schatz DA, Peck AB, Cornelius JG: Reversal of insulin-dependent diabetes using islets generated in vitro from pancreatic stem cells. Nat Med 2000, 6:278-282 [DOI] [PubMed] [Google Scholar]
- 15.Overturf K, Al-Dhalimy M, Tanguay R, Brantly M, Ou CN, Finegold M, Grompe M: Hepatocytes corrected by gene therapy are selected in vivo in a murine model of hereditary tyrosinaemia type I. Nat Genet 1996, 12:266-273 [DOI] [PubMed] [Google Scholar]
- 16.Overturf K, Al-Dhalimy M, Ou CN, Finegold M, Grompe M: Serial transplantation reveals the stem-cell-like regenerative potential of adult mouse hepatocytes. Am J Pathol 1997, 151:1273-1280 [PMC free article] [PubMed] [Google Scholar]
- 17.Overturf K, Al-Dhalimy M, Finegold M, Grompe M: The repopulation potential of hepatocyte populations differing in size and prior mitotic expansion. Am J Pathol 1999, 155:2135-2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grompe M, Al-Dhalimy M, Finegold M, Ou CN, Burlingame T, Kennaway NG, Soriano P: Loss of fumarylacetoacetate hydrolase is responsible for the neonatal hepatic dysfunction phenotype of lethal albino mice. Genes Dev 1993, 7:2298-2307 [DOI] [PubMed] [Google Scholar]
- 19.Grompe M, Laconi E, Shafritz DA: Principles of therapeutic liver repopulation. Semin Liver Dis 1999, 19:7-14 [DOI] [PubMed] [Google Scholar]
- 20.Friedrich G, Soriano P: Promoter traps in embryonic stem cells: a genetic screen to identify and mutate developmental genes in mice. Genes Dev 1991, 5:1513-1523 [DOI] [PubMed] [Google Scholar]
- 21.Lindstedt S, Holme E, Lock EA, Hjalmarson O, Strandvik B: Treatment of hereditary tyrosinaemia type I by inhibition of 4-hydroxyphenylpyruvate dioxygenase. Lancet 1992, 340:813-817 [DOI] [PubMed] [Google Scholar]
- 22.Grompe M, Lindstedt S, Al-Dhalimy M, Kennaway NG, Papaconstantinou J, Torres-Ramos CA, Ou CN, Finegold M: Pharmacological correction of neonatal lethal hepatic dysfunction in a murine model of hereditary tyrosinaemia type I. Nat Genet 1995, 10:453-460 [DOI] [PubMed] [Google Scholar]
- 23.Githens S, Schexnayder JA, Moses RL, Denning GM, Smith JJ, Frazier ML: Mouse pancreatic acinar/ductular tissue gives rise to epithelial cultures that are morphologically, biochemically, and functionally indistinguishable from interlobular duct cell cultures. In Vitro Cell Dev Biol Anim 1994, 30:622-635 [DOI] [PubMed] [Google Scholar]
- 24.Richards J, Larson L, Yang J, Guzman R, Tomooka Y, Osborn R, Nandis WI: Method for culturing mammary epithelial cells in a rat tail collagen gel matrix. J Tissue Culture Methods 1983, 8:31-36 [Google Scholar]
- 25.Sturman JA, Applegarth DA: Automated amino acid analysis. Neuromethods, 1985, vol. 3. GB Baker, JD Wood. Totowa, NJ, Humana Press Inc., Edited by AA Boulton
- 26.Grenier A, Lescault A: Succinylacetone. Bergmeyer HU eds. Methods of Enzymatic Analysis. 1985, :p 79 VCH Verlagsgesellschaft, Weinheim [Google Scholar]
- 27.Knox WE, Edwards SW: Enzymes involved in conversion of tyrosine to acetoacetate. Methods Enzymol 1955, 2:287-300 [Google Scholar]
- 28.Bradford MM: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976, 72:248-254 [DOI] [PubMed] [Google Scholar]
- 29.Smith DDJ, Campbell JW: Distribution of glutamine synthetase and carbamoyl-phosphate synthetase I in vertebrate liver. Proc Natl Acad Sci USA 1988, 85:160-164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eckert JW, Buerkle CJ, Major AM, Finegold MJ, Brandt ML: In situ hybridization utilizing a Y chromosome DNA probe. Use as a cell marker for hepatocellular transplantation. Transplantation 1995, 59:109-111 [DOI] [PubMed] [Google Scholar]
- 31.Sambrook J, Fritsch EF, Maniatis T: Molecular Cloning: A Laboratory Manual. 1989. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
- 32.Overturf K, Al-Dhalimy M, Ou CN, Finegold M, Tanguay R, Lieber A, Kay M, Grompe M: Adenovirus-mediated gene therapy in a mouse model of hereditary tyrosinemia type I. Hum Gene Ther 1997, 8:513-521 [DOI] [PubMed] [Google Scholar]
- 33.Grompe M, Overturf K, Al-Dhalimy M, Finegold M: Therapeutic trials in the murine model of hereditary tyrosinaemia type I: a progress report. J Inherit Metab Dis 1998, 21:518-531 [DOI] [PubMed] [Google Scholar]
- 34.Githens S: Pancreatic duct cell cultures. Annu Rev Physiol 1994, 56:419-443 [DOI] [PubMed] [Google Scholar]
- 35.Bisgaard HC, Thorgeirsson SS: Evidence for a common cell of origin for primitive epithelial cells isolated from rat liver and pancreas. J Cell Physiol 1991, 147:333-343 [DOI] [PubMed] [Google Scholar]
- 36.Bonner-Weir S, Taneja M, Weir GC, Tatarkiewicz K, Song KH, Sharma A, O’Neil JJ: In vitro cultivation of human islets from expanded ductal tissue. Proc Natl Acad Sci USA 2000, 97:7999-8004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sell S, Ilic Z: Liver Stem Cells. 1997. TX, R.G. Landes, Austin
- 38.Thorgeirsson SS: Hepatic stem cells in liver regeneration. FASEB J 1996, 10:1249-1256 [PubMed] [Google Scholar]